Abstract
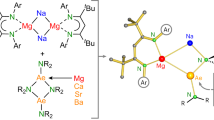
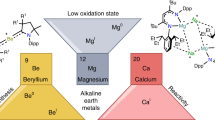
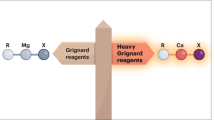
A complex of a metal in its zero oxidation state can be considered a stabilized, but highly reactive, form of a single metal atom. Such complexes are common for the more noble transition metals. Although rare examples are known for electronegative late-main-group p-block metals or semimetals1,2,3,4,5,6, it is a challenge to isolate early-main-group s-block metals in their zero oxidation state7,8,9,10,11. This is directly related to their very low electronegativity and strong tendency to oxidize. Here we present examples of zero-oxidation-state magnesium (that is, magnesium(0)) complexes that are stabilized by superbulky, monoanionic, β-diketiminate ligands. Whereas the reactivity of an organomagnesium compound is typically defined by the nucleophilicity of its organic groups and the electrophilicity of Mg2+ cations, the Mg0 complexes reported here feature electron-rich Mg centres that are nucleophilic and strongly reducing. The latter property is exemplified by the ability to reduce Na+ to Na0. We also present a complex with a linear Mg3 core that formally could be described as a MgI–Mg0–MgI unit. Such multinuclear mixed-valence Mgn clusters are discussed as fleeting intermediates during the early stages of Grignard reagent formation. Their remarkably strong reducing power implies a rich reactivity and application as specialized reducing agents.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
X-ray data are available free of charge from the Cambridge Crystallographic Data Centre under references CCDC 2045616 (1), 2045617 (2), 2045618 (3), 2045619 (4) and 2045620 (5). Spectroscopic data that support the findings of this study as well as complementary crystallographic and computational details are included in Supplementary Information. Raw data are available from the corresponding author on reasonable request.
References
Wang, Y. et al. A stable silicon(0) compound with a Si=Si double bond. Science 321, 1069–1071 (2008).
Sidiropoulos, A., Jones, C., Stasch, A., Klein, S. & Frenking, G. N-Heterocyclic carbene stabilized digermanium(0). Angew. Chem. Int. Edn 48, 9701–9704 (2009).
Braunschweig, H. et al. Ambient-temperature isolation of a compound with a boron-boron triple bond. Science 336, 1420–1422 (2012).
Jones, C., Sidiropoulos, A., Holzmann, N., Frenking, G. & Stasch, A. An N-heterocyclic carbene adduct of diatomic tin, :Sn=Sn:. Chem. Commun. 48, 9855–9857 (2012).
Mondal, K. C. et al. A stable singlet biradicaloid siladicarbene: (L:)2Si. Angew. Chem. Int. Edn 52, 2963–2967 (2013).
Li, Y. et al. Acyclic germylones: congeners of allenes with a central germanium atom. J. Am. Chem. Soc. 135, 12422–12428 (2013).
Glaunsinger, W. S. et al. Structure and molecular motions in alkaline earth hexammines. Nature 271, 414–417 (1978).
Wu, X. et al. Observation of alkaline earth complexes M(CO)8 (M = Ca, Sr, or Ba) that mimic transition metals. Science 361, 912–916 (2018).
Wang, Q. et al. Transition-metal chemistry of alkaline-earth elements: the trisbenzene complexes M(Bz)3 (M=Sr, Ba). Angew. Chem. Int. Edn 58, 17365–17374 (2019).
Arrowsmith, M. et al. Neutral zero-valent s-block complexes with strong multiple bonding. Nat. Chem. 8, 890–894 (2016).
Couchman, S. A., Holzmann, N., Frenking, G., Wilson, D. J. D. & Dutton, J. L. Beryllium chemistry the safe way: a theoretical evaluation of low oxidation state beryllium compounds. Dalton Trans. 42, 11375–11384 (2013).
Green, S. P., Jones, C. & Stasch, A. Stable magnesium(I) compounds with Mg–Mg bonds. Science 318, 1754–1757 (2007).
Jones, C. Dimeric magnesium(I) β-diketiminates: a new class of quasi-universal reducing agent. Nat. Rev. Chem. 1, 0059 (2017).
Jones, C. Open questions in low oxidation state group 2 chemistry. Commun. Chem. 3, 159 (2020).
Gentner, T. X. et al. Low valent magnesium chemistry with a super bulky β‐diketiminate ligand. Angew. Chem. Int. Edn 58, 607–611 (2019).
Jones, D. D. L., Douair, I., Maron, L. & Jones, C. Photochemically activated dimagnesium(I) compounds: reagents for the reduction and selective C–H bond activation of inert arenes. Angew. Chem. Int. Edn 60, 7087–7092 (2021).
Rösch, B. et al. Mg-Mg bond polarization induced by a superbulky β-diketiminate ligand. Chem. Commun. 56, 11402–11405 (2020).
Hicks, J., Juckel, M., Paparo, A., Dange, D. & Jones, C. Multigram syntheses of magnesium(I) compounds using alkali metal halide supported alkali metals as dispersible reducing agents. Organometallics 37, 4810–4813 (2018).
Cui, C. et al. Synthesis and structure of a monomeric aluminum(I) compound [{HC(CMeNAr)2}Al] (Ar=2,6-iPr2C6H3): a stable aluminum analogue of a carbene. Angew. Chem. Int. Edn 39, 4274–4276 (2000).
Hicks, J., Vasko, P., Goicoechea, J. M. & Aldridge, S. Synthesis, structure and reaction chemistry of a nucleophilic aluminyl anion. Nature 557, 92–95 (2018); correction 560, E24 (2018).
Dye, J. L., Ceraso, J. M., Lok Tak, M., Barnett, B. L. & Tehan, F. J. Crystalline salt of the sodium anion (Na–). J. Am. Chem. Soc. 96, 608–609 (1974).
Camp, C. & Arnold, J. On the non-innocence of “NacNacs”: ligand-based reactivity in β-diketiminate supported coordination compounds. Dalton Trans. 45, 14462–14498 (2016).
Slater, J. C. Atomic radii in crystals. J. Chem. Phys. 41, 3199–3204 (1964).
Lambert, C. & von Ragué Schleyer, P. Are polar organometallic compounds “carbanions”? The gegenion effect on structure and energies of alkali-metal compounds. Angew. Chem. Int. Edn Engl. 33, 1129–1140 (1994).
Cao, W. L., Gatti, C., MacDougall, P. J. & Bader, R. F. W. On the presence of non-nuclear attractors in the charge distributions of Li and Na clusters. Chem. Phys. Lett. 141, 380–385 (1987).
Gentner, T. X. & Mulvey, R. E. Alkali metal mediation: diversity of applications in main group organometallic chemistry. Angew. Chem. Int. Edn 59, 2–18 (2020).
Arrowsmith, M. et al. Mononuclear three-coordinate magnesium complexes of a highly sterically encumbered β-diketiminate ligand. Inorg. Chem. 53, 10543–10552 (2014).
Bakewell, C., White, A. J. P. & Crimmin, M. R. Addition of carbon-fluorine bonds to a Mg(I)-Mg(I) bond: an equivalent of Grignard formation in solution. J. Am. Chem. Soc. 138, 12763–12766 (2016).
Hicks, J., Underhill, E. J., Kefalidis, C. E., Maron, L. & Jones, C. A mixed-valence tri-zinc complex, [LZnZnZnL] (L=bulky amide), bearing a linear chain of two-coordinate zinc atoms. Angew. Chem. Int. Edn 54, 10000–10004 (2015).
Bakewell, C., Ward, B. J., White, A. J. P. & Crimmin, M. R. A combined experimental and computational study on the reaction of fluoroarenes with Mg-Mg, Mg-Zn, Mg-Al and Al-Zn bonds. Chem. Sci. 9, 2348–2356 (2018).
Platts, J. A., Overgaard, J., Jones, C., Iversen, B. B. & Stasch, A. First experimental characterization of a non-nuclear attractor in a dimeric magnesium(I) compound. J. Phys. Chem. A 115, 194–200 (2011).
Garst, G. F. & Ungvary, F. in Grignard Reagents: New Developments (ed. Richey, H.) 185–276 (Wiley, 2000).
Tjurina, L. A. et al. Synthesis of cluster alkyl and aryl Grignard reagents in solution. Organometallics 23, 1349–1351 (2004).
Imizu, Y. & Klabunde, K. J. Metal cluster vs. atom reactivities: magnesium cluster Grignard reagents. Inorg. Chem. 23, 3602–3605 (1984).
Köhn, A., Weigend, F. & Ahlrichs, R. Theoretical study on clusters of magnesium. Phys. Chem. Chem. Phys. 3, 711–719 (2001).
Eriksson, L. A. Accurate density functional theory study of cationic magnesium clusters and Mg+-rare gas interactions. J. Chem. Phys. 103, 1050–1056 (1995).
Kruczyński, T., Henke, F., Neumaier, M., Bowen, K. H. & Schnöckel, H. Many Mg-Mg bonds form the core of the Mg16Cp*8Br4K cluster anion: the key to a reassessment of the Grignard reagent (GR) formation process? Chem. Sci. 7, 1543–1547 (2016).
Velazquez, A., Fernández, I., Frenking, G. & Merino, G. Multimetallocenes. A theoretical study. Organometallics 26, 4731–4736 (2007).
Chase, M. W. Jr NIST-JANAF Thermochemical Tables Part 2, 4th edn (Monograph No. 9, J. Phys. Chem. Ref. Data, American Institution of Physics, 1998).
Clegg, W. et al. Synthesis and structures of [(trimethylsilyl)methyl]sodium and ‐potassium with bi‐ and tridentate N‐donor ligands. Eur. J. Inorg. Chem. 721–726 (2011).
Rigaku Oxford Diffraction. CrysAlisPro Software system, version 1.171.40.67a (Rigaku Corporation, 2018).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42, 339–341 (2009).
Sheldrick, G. M. SHELXT — Integrated space-group and crystal-structure determination. Acta Crystallogr. A 71, 3–8 (2015).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 71, 3–8 (2015).
Frisch, M. J. et al. Gaussian 16 Rev. A.03 (Gaussian, Inc., 2016).
Becke, A. D. A new mixing of Hartree–Fock and local density‐functional theories. J. Chem. Phys. 98, 1372–1377 (1993).
Perdew, J. P. Electronic Structure of Solids (Akademie, 1991).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Reed, A. E., Weinstock, R. B. & Weinhold, F. Natural population analysis. J. Chem. Phys. 83, 735–746 (1985).
Bader, R. F. W. A quantum theory of molecular structure and its applications. Chem. Rev. 91, 893–928 (1991).
Keith, T. A. AIMAll Version 17.01.25 (TK Gristmill Software, 2017).
Acknowledgements
We thank A. Roth (FAU) and the Kolbe Microanalytical Laboratory (Mülheim/Ruhr) for elemental analysis. This work was generously supported by the University of Erlangen-Nürnberg.
Author information
Authors and Affiliations
Contributions
B.R.: conceptualization, investigation, validation, formal analysis (that is, analyses of raw data from spectroscopy, X-ray diffraction or computational methods), writing of original draft and visualization. T.X.G.: investigation, validation and formal analysis. J.E.: formal analysis. J.L.: formal analysis. H.E.: formal analysis. S.H.: conceptualization, writing of original draft, review and editing, visualization, supervision and project administration.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Jason Dutton and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains: Spectroscopic characterization (including Supplementary Figs. 1 to 41); Crystal structure determination (including Supplementary Figs. 42 to 46 and Supplementary Tables 1 to 5); Comparison of chemical shifts and bond distances for (BDI*)Mg complexes (including Supplementary Table 6); Computational details (including Supplementary Figs. 47 to 57 and Supplementary Tables 7-15); Arguments in favor for the correct metal assignment in {[(BDI*)Mg−][Na+]}2 (1); and Supplementary References.
Rights and permissions
About this article
Cite this article
Rösch, B., Gentner, T.X., Eyselein, J. et al. Strongly reducing magnesium(0) complexes. Nature 592, 717–721 (2021). https://doi.org/10.1038/s41586-021-03401-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03401-w
This article is cited by
-
Reduction of Li+ within a borate anion
Nature Communications (2024)
-
Achieving unusual metal–metal bonding in the s-block
Nature Synthesis (2024)
-
Reactivity of the magnesium bisamide complex towards C=C=O-, N=C=O-, and N—N=O-containing substrates
Russian Chemical Bulletin (2024)
-
Alkali metal reduction of alkali metal cations
Nature Communications (2023)
-
Heterobimetallic alkaline earth metal–metal bonding
Nature Synthesis (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.