Abstract
Upon gamete fusion, animal egg cells secrete proteases from cortical granules to establish a fertilization envelope as a block to polyspermy1,2,3,4. Fertilization in flowering plants is more complex and involves the delivery of two non-motile sperm cells by pollen tubes5,6. Simultaneous penetration of ovules by multiple pollen tubes (polytubey) is usually avoided, thus indirectly preventing polyspermy7,8. How plant egg cells regulate the rejection of extra tubes after successful fertilization is not known. Here we report that the aspartic endopeptidases ECS1 and ECS2 are secreted to the extracellular space from a cortical network located at the apical domain of the Arabidopsis egg cell. This reaction is triggered only after successful fertilization. ECS1 and ECS2 are exclusively expressed in the egg cell and transcripts are degraded immediately after gamete fusion. ECS1 and ESC2 specifically cleave the pollen tube attractor LURE1. As a consequence, polytubey is frequent in ecs1 ecs2 double mutants. Ectopic secretion of these endopeptidases from synergid cells led to a decrease in the levels of LURE1 and reduced the rate of pollen tube attraction. Together, these findings demonstrate that plant egg cells sense successful fertilization and elucidate a mechanism as to how a relatively fast post-fertilization block to polytubey is established by fertilization-induced degradation of attraction factors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Published RNA sequencing data (Gene Expression Omnibus (GEO) accession numbers GSE121003, GSE33713, GSE32318, GSE102694 and GSE87760) were used for expression analysis in the present study. The raw data for the graphs that support the findings of this study are available online, and uncropped gel images are shown in the Supplementary Information file. The seeds of the transgenic lines described in this report are available from the corresponding authors on request. Source data are provided with this paper.
References
Wong, J. L. & Wessel, G. M. Defending the zygote: search for the ancestral animal block to polyspermy. Curr. Top. Dev. Biol. 72, 1–151 (2006).
Burkart, A. D., Xiong, B., Baibakov, B., Jiménez-Movilla, M. & Dean, J. Ovastacin, a cortical granule protease, cleaves ZP2 in the zona pellucida to prevent polyspermy. J. Cell Biol. 197, 37–44 (2012).
Vacquier, V. D., Tegner, M. J. & Epel, D. Protease activity establishes the block against polyspermy in sea urchin eggs. Nature 240, 352–353 (1972).
Liu, M. The biology and dynamics of mammalian cortical granules. Reprod. Biol. Endocrinol. 9, 149 (2011).
Johnson, M. A., Harper, J. F. & Palanivelu, R. A fruitful journey: pollen tube navigation from germination to fertilization. Annu. Rev. Plant Biol. 70, 809–837 (2019).
Dresselhaus, T., Sprunck, S. & Wessel, G. M. Fertilization mechanisms in flowering plants. Curr. Biol. 26, R125–R139 (2016).
Beale, K. M., Leydon, A. R. & Johnson, M. A. Gamete fusion is required to block multiple pollen tubes from entering an Arabidopsis ovule. Curr. Biol. 22, 1090–1094 (2012).
Maruyama, D. et al. Independent control by each female gamete prevents the attraction of multiple pollen tubes. Dev. Cell 25, 317–323 (2013).
Zhang, J. et al. Sperm cells are passive cargo of the pollen tube in plant fertilization. Nat. Plants 3, 17079 (2017).
Glöckle, B. et al. Pollen differentiation as well as pollen tube guidance and discharge are independent of the presence of gametes. Development 145, dev152645 (2018).
Zhou, L. Z. & Dresselhaus, T. Friend or foe: signaling mechanisms during double fertilization in flowering seed plants. Curr. Top. Dev. Biol. 131, 453–496 (2019).
Grossniklaus, U. Polyspermy produces tri-parental seeds in maize. Curr. Biol. 27, R1300–R1302 (2017).
Nakel, T. et al. Triparental plants provide direct evidence for polyspermy induced polyploidy. Nat. Commun. 8, 1033 (2017).
Márton, M. L., Cordts, S., Broadhvest, J. & Dresselhaus, T. Micropylar pollen tube guidance by egg apparatus 1 of maize. Science 307, 573–576 (2005).
Takeuchi, H. & Higashiyama, T. A species-specific cluster of defensin-like genes encodes diffusible pollen tube attractants in Arabidopsis. PLoS Biol. 10, e1001449 (2012).
Okuda, S. et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature 458, 357–361 (2009).
Zhong, S. et al. Cysteine-rich peptides promote interspecific genetic isolation in Arabidopsis. Science 364, eaau9564 (2019).
Sandaklie-Nikolova, L., Palanivelu, R., King, E. J., Copenhaver, G. P. & Drews, G. N. Synergid cell death in Arabidopsis is triggered following direct interaction with the pollen tube. Plant Physiol. 144, 1753–1762 (2007).
Duan, Q. et al. FERONIA controls pectin- and nitric oxide-mediated male-female interaction. Nature 579, 561–566 (2020).
Maruyama, D. et al. Rapid elimination of the persistent synergid through a cell fusion mechanism. Cell 161, 907–918 (2015).
Kasahara, R. D. et al. Fertilization recovery after defective sperm cell release in Arabidopsis. Curr. Biol. 22, 1084–1089 (2012).
Sprunck, S. et al. Egg cell-secreted EC1 triggers sperm cell activation during double fertilization. Science 338, 1093–1097 (2012).
Simões, I., Faro, R., Bur, D. & Faro, C. Characterization of recombinant CDR1, an Arabidopsis aspartic proteinase involved in disease resistance. J. Biol. Chem. 282, 31358–31365 (2007).
Mori, T., Kuroiwa, H., Higashiyama, T. & Kuroiwa, T. GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nat. Cell Biol. 8, 64–71 (2006).
Mori, T., Igawa, T., Tamiya, G., Miyagishima, S. Y. & Berger, F. Gamete attachment requires GEX2 for successful fertilization in Arabidopsis. Curr. Biol. 24, 170–175 (2014).
Steffen, J. G., Kang, I. H., Macfarlane, J. & Drews, G. N. Identification of genes expressed in the Arabidopsis female gametophyte. Plant J. 51, 281–292 (2007).
Bleckmann, A. & Dresselhaus, T. Whole mount RNA-FISH on ovules and developing seeds. Methods Mol. Biol. 1669, 159–171 (2017).
Zimmerberg, J. & Whitaker, M. Irreversible swelling of secretory granules during exocytosis caused by calcium. Nature 315, 581–584 (1985).
Antoine, A. F. et al. A calcium influx is triggered and propagates in the zygote as a wavefront during in vitro fertilization of flowering plants. Proc. Natl Acad. Sci. USA 97, 10643–10648 (2000).
Digonnet, C., Aldon, D., Leduc, N., Dumas, C. & Rougier, M. First evidence of a calcium transient in flowering plants at fertilization. Development 124, 2867–2874 (1997).
Denninger, P. et al. Male–female communication triggers calcium signatures during fertilization in Arabidopsis. Nat. Commun. 5, 4645 (2014).
Hamamura, Y. et al. Live imaging of calcium spikes during double fertilization in Arabidopsis. Nat. Commun. 5, 4722 (2014).
Kranz, E., von Wiegen, P. & Lörz, H. Early cytological events after induction of cell division in egg cells and zygote development following in vitro fertilization with angiosperm gametes. Plant J. 8, 9–23 (1995).
Wu, J. J. et al. Mitochondrial GCD1 dysfunction reveals reciprocal cell-to-cell signaling during the maturation of Arabidopsis female gametes. Dev. Cell 23, 1043–1058 (2012).
Lampropoulos, A. et al. GreenGate—a novel, versatile, and efficient cloning system for plant transgenesis. PLoS ONE 8, e83043 (2013).
Nelson, B. K., Cai, X. & Nebenführ, A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 51, 1126–1136 (2007).
Zhang, X., Henriques, R., Lin, S. S., Niu, Q. W. & Chua, N. H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 1, 641–646 (2006).
Wang, T. et al. A receptor heteromer mediates the male perception of female attractants in plants. Nature 531, 241–244 (2016).
Sparkes, I. A., Runions, J., Kearns, A. & Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 1, 2019–2025 (2006).
Soares, A. et al. An atypical aspartic protease modulates lateral root development in Arabidopsis thaliana. J. Exp. Bot. 70, 2157–2171 (2019).
Zhao, P. et al. Two-step maternal-to-zygotic transition with two-phase parental genome contributions. Dev. Cell 49, 882–893 (2019).
Acknowledgements
We thank F. Berger for the HTR10–mRFP marker line, Y. Zhang for the LAT52::DsRed maker line, C. Li for the LAT52::GUS maker line and W. Li for help with the mammal cell expression system. This work was supported by the National Natural Science Foundation of China (31991201) and the German Research Council DFG via SFB960.
Author information
Authors and Affiliations
Contributions
M.-x.S., X.Y. and T.D. designed the research plan. X.Z., X.P., H.C. and C.S. performed the phenotype and genetics analyses. X.Y. and P.Z. performed the biochemical study. A. Bazhenova performed the in situ hybridization study. A. Bleckmann performed the cortical network observation study. T.D. and M.-x.S. contributed to the data analysis and finalized the manuscript. All authors contributed to the data collection, presentation and manuscript writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
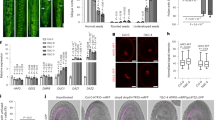
Extended Data Fig. 1 ECS1 and ECS2 are specifically expressed in the egg cell of Arabidopsis.
a, Fragments per kilobase of transcript per million mapped reads (FPKM) values (Mean ± s.d.) of ECS1 and ECS2 transcripts in egg cells and zygotes. RNA-seq data of Ec, Zy 1C and 32C are from ref. 41 except 8C (GSE33713), seedlings (GSE32318), stems (GSE102694), roots and rosettes (GSE87760). b–i, Promoter activity analysis using the nuclear marker H2B–GFP expressed by ECS1 (b–e) and ECS2 (f–i) promoters, respectively. Both promoters were specifically active in egg cells after embryo sac cellularization. b, f, Female gametophyte before cellularization. c, g, Immature egg cell. d, h, Mature egg cell. e, i, Zygote at 20 HAP. 1C, 1-cell pro-embryo; 8C, 8-cell pro-embryo; 32C, 32-cell embryo; n, nucleus; ecn, egg cell nucleus; syn, synergid cell nucleus; zy, zygote. Dashed lines outline the egg cell and zygote, respectively. Insets show enlargements of regions indicated. Scale bars, 20 μm.
Extended Data Fig. 2 Characteristics of ECS protein sequences, identification of their T-DNA insertion mutants and phylogenetic tree of aspartic endopeptidases in Arabidopsis.
a, Alignment of ECS1/2 and CDR1 protein sequences. Two active sites and an N-terminal signal peptide are indicated. Conserved cysteines typical for aspartic proteases are labelled in yellow. b, Protein sequences of 78 aspartic proteases from A. thaliana annotated in the MEROPS database (https://merops.sanger.ac.uk/) were subjected to phylogenetic analysis by MEGA X. The phylogenetic tree was constructed using the Neighbour-Joining method. ECS1 and ECS2 are indicated in red. c, Scheme showing T-DNA insertion sites of ecs1 and ecs2 mutants. d, RT–PCR using primers indicated in b revealed that transcripts levels of ECS 1 and ECS2 were significantly reduced in their corresponding T-DNA insertion mutants. Actin was used as a control for RT–PCR analysis.
Extended Data Fig. 3 As a consequence of polytubey, multiple sperm cell pairs are released in ecs1 ecs2 mutant ovules.
a, Three representative images showing two additional sperm cell pairs at 24 HAP, respectively. b–d, Time series showing representative images of two additional sperm cell pairs at 6 HAP (b), 8 HAP (c) and10 HAP (d), respectively. e, Proportions of additional sperm pairs in ovules of ecs1 ecs2 mutants after fertilization (n = 1056 for 6 HAP; 1114 for 8 HAP, 1083 for 10 HAP). Data are presented in box-and-whisker plots. Bottom and top of the box, 25th and 75th percentiles; centre line, 50th percentile; whiskers, minimum and maximum data. Abbreviations: sp., sperm cell; enn, endosperm nucleus; zyn, zygote nucleus. Scale bars, 20 μm.
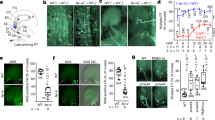
Extended Data Fig. 4 ECS1 and ECS2 are almost quantitatively secreted from an apical network of the mature egg cell to the extracellular space only after gamete fusion.
a, b, ECS1-mCitrine (mCit) at the apical domain forming a network before fertilization. c, d, ECS2–mCit accumulate at the apical domain forming a network before fertilization. b, d, Enlargement of apical domains of egg cells shown in a, b. e–h, ECS1–mCit (e, f) and ECS2–mCit (g, h) are secreted from the egg cell to the extracellular space. Synergids are largely degenerated as indicated by the lack or diminished signal of the synergid marker. Volume projections of z-stacks from ECS1/2–mCit (yellow), an egg cell expressed Golgi–mScarlet (mScar; red) and a synergid expressed endoplasmic reticulum marker tagged to mTurquoise2 (mTur; cyan) are shown. i, FM4-64 staining showing that the cortical network containing ECS2–mCit is located at the plasma membrane. j, Signal intensity plot along the arrow shown in i indicates that ECS2–Cit is located at or just below the plasma membrane. k, Single optical section through the cortical network shows weak ECS2–Cit signals outside the network. l, Overexposure of the same optical section shown in k illustrates ECS2–Cit signals throughout the egg cytoplasm. m, Overexposed image showing ECS2–GFP accumulating in the apical egg cell domain and the endoplasmic reticulum maker EC1.2::erRFP marking the boundaries of the egg cell. A pollen tube expressing DsRed driven by the Lat52 promoter was used to monitor pollen tube perception. During pollen tube arrival ECS2–GFP was not yet released. n, Intensity plot profile showing relative fluorescence signal intensities of ECS2–GFP (green line) and erRFP (red line) along a dashed line drawn across the egg cells (indicated in the left image) confirming the microscopic observation. o, Sperm cells defective in gamete fusion (gcs1 mutant) did not trigger ECS2–GFP release. p, Intensity plot profile as in n showing that egg cell-localized ECS2–GFP and synergid cell-localized erRFP signals do not overlap. Ec, egg cell; pt, pollen tube; sp., sperm cells; sy, synbergid cells. Scale bars, 10 μm.
Extended Data Fig. 5 Truncated ECS1 and ECS2 proteins are not secreted from egg cells during fertilization.
a, d, ECS1–GFP and ECS2–GFP were located inside the egg cell before fertilization. b, e, ECS1–GFP and ECS2–GFP were secreted from the egg cell at 8 HAP. Asterisks mark secreted ECS1–GFP and ECS2–GFP, respectively. c, f, Truncated TECS1–GFP (c) and TECS2–GFP (f) versions lacking signal peptides could not be secreted from the egg cell at 8 HAP. ec, egg cell. Dashed lines outline the egg cell boundaries. Insets show enlargements of regions indicated. Scale bars, 20 μm.
Extended Data Fig. 6 ECS1 and ECS2 endopeptidases interact with LURE1.2 and cleave it as a substrate.
a, Protein level of LURE1.2 significantly decreased after co-expression with ECS1 and ECS2 in mammalian cells, respectively. b, Relative protein levels of LURE1.2 in leaves co-expressed with ECS1, ECS2 or the empty vector (CK) as control, respectively. Data are presented as mean ± s.d. from four independent experiments. (n = 4). Statistical test was performed using one-way ANOVA between groups, with the Tukey–Kramer test for multiple comparisons (P = 2.25 × 10−5; F = 44.03). c, Fluorogenic peptides were synthesized according to the LURE1.2 protein sequence (d). e, Proteolytic activity of recombinant ECS1 and ECS2 using LURE1.2-derived peptide 3 as substrate at different concentrations to determine Km values. Data represent mean ± s.d. of three independent experiments. f, Effect of temperature on proteolytic activities of recombinant ECS1 and ECS2 using peptide 3 as a substrate. Data represent mean ± s.d. of three independent experiments. g, Alignment of LURE and XIUQIU protein sequences. Amino acid sequence of peptide 3 (outlined by red colour) is conserved in all LURE1, but not in XIUQIU protein sequences. Full length sequences including N-terminal signal motifs are shown.
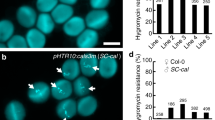
Extended Data Fig. 7 ECS1 and ECS2 efficiently cleave LURE1 substrates.
a, Localization and protein level of LURE1.2–GFP before pollination and in fertilized ovules of WT and ecs1 ecs2 mutant pistils, respectively. Pistils were pollinated with pollen expressing HTR10–mRFP in sperm cells. Ovules were collected from pistils at 0 and 10 HAP. ecs1 ecs2 mutation resulted in the accumulation of LURE1.2 after fertilization. b, Quantification of green fluorescence intensity in ovules from WT and ecs1 ecs2 pistils (n = 101). Data for fluorescence intensity are presented in box-and-whisker plots. Bottom and top of the box, 25th and 75th percentiles; centre line, 50th percentile; whiskers, minimum and maximum data. ** indicates statistically significant difference between WT and mutant ovules (two-tailed Student’s t-test; P < 0.01). c, In vitro pollen tube attraction assay with gelatin beads containing ECS1- and ECS2-digested LURE1.2, respectively. Beads (*) were prepared using 1 μM LURE1.2 alone and in combination with 1 μM ECS1 and ECS2, respectively, and placed close to growing pollen tube tips (0 min) and observed for 60 min. Pollen tube attraction activity was lost when beads contained both, LURE1.2 and ECS endopeptidases. n, nucleus; pt, pollen tube; sy, synergid cell; zy, zygote. Scale bars are 10 μm (a) and 50 μm (c).
Extended Data Fig. 8 Ectopic expression of ECS1 and ECS2 in synergid cells leads to a decrease of LURE1.2 protein levels and strongly reduced pollen tube attraction rate.
a, Ectopically expressed ECS1–GFP fusion protein in synergid cells is secreted to the filiform apparatus. b, Immunofluorescence revealed that LURE1 levels were significant decreased in ovules ectopically expressing ECS1-GFP in synergid cells using the DD31 promoter. c, Quantification of LURE1 fluorescence intensity in WT and ECS1-ectopically expressed ovules as shown in (b) (n = 10). d, Ectopic expression of ECS1 in synergid cells resulted in pollen tube attraction defects 6 HAP. Pollen tube growth analysis was performed using a Lat52::GUS reporter line. e, Percentages of ovules attracting pollen tubes observed in WT pistils and those ectopically expressing ECS1 in synergid cells of three independent lines (L1–L3) at 6 HAP (n = 50 for WT and DD31::ECS1 L1; 60 for DD31::ECS1 L2 and DD31::ECS1 L3). f, Immunofluorescence revealed that LURE1 levels were significantly decreased in ovules ectopically expressing ECS2 in synergid cells using the DD31 promoter. g, Quantification of LURE1 fluorescence intensity in WT ovules and those ectopically expressing ECS2 (n = 10). h, Similarly, LURE1.2–GFP signals were significantly decreased in ovules ectopically expressing ECS2 in synergid cells. i, Quantification of LURE1.2–GFP fluorescence intensity as described in b (n = 101). j, k, Ectopic expression of ECS2 in synergid cells resulted in reduction of pollen tube attraction 6 HAP. Lat52::DsRed (j) and Lat52::GUS reporter line (k) were used in this analysis. l, Proportions of ovules attracting pollen tubes observed in WT plants and those ectopically expressing ECS2 in synergid cells at 6 HAP (n = 50 for WT, DD31::ECS2 L2 and DD31::ECS2 L3; 80 for DD31::ECS2 L1). Data in c, g, i are presented in box-and-whisker plots. Bottom and top of the box, 25th and 75th percentiles; centre line, 50th percentile; whiskers, minimum and maximum data. ** indicates statistical difference compared to WT (Two-tailed Student’s t-test; P < 0.01). Same letters (in e and l) indicate lack of significant differences according to the Tukey–Kramer multiple comparison test (one-way ANOVA between groups; P = 1.94 × 10−7; F = 32.11 in e; P = 3.24 × 10−8, F = 37.88 in l). P < 0.05 was considered as significant. Scale bars, 20 μm (a, b, f, h), 100 μm (j). ec, egg cell; sy, synergid; pt, pollen tube; ov, ovule.
Extended Data Fig. 9 Ectopic expression of truncated versions of ECS1 and ECS2 or the subtilisin-like protease SBT4.13 in synergid cells have no significant influence on LURE1.2 protein levels and pollen tube attraction rate.
a, Ectopic expressions of truncated versions of ECS1 and ECS2 (TECS1/2) in synergid cells. b, Proportions of ovules attracting pollen tubes observed in WT plants and those ectopically expressing TECS1/2 in synergid cells at 6 HAP (n = 50). c, Immunofluorescence revealed that LURE1 levels were comparable in WT and ovules ectopically expressing TECS1/2 in synergid cells using the DD31 promoter. d, Quantification of LURE1 fluorescence intensity in WT ovules and those ectopically expressing truncated TECS1/2 in synergid cells (n = 10). e, Ectopic expressions of the egg cell expressed subtilisin-like protease SBT4.1327 as a GFP fusion protein in synergid cells. SBT4.13–GFP is secreted to the filiform apparatus. f, Proportions of ovules attracting pollen tubes observed in WT plants and those ectopically expressing SBT4.13 in synergid cells at 6 HAP (n = 50). g, Immunofluorescence revealed that LURE1 levels were comparable in WT and ovules ectopically expressing SBT4.13 in synergid cells. h, Quantification of LURE1 fluorescence intensity in WT ovules and those ectopically expressing SBT4.13 (n = 10). Data in b, f represent the mean ± s.d. Same letters in b, f indicate lack of significant differences according to the Tukey–Kramer multiple comparison test (one-way ANOVA between groups; P = 0.96, F = 0.04 in b left plane; P = 0.85, F = 0.17 in b right plane; P = 0.85, F = 0.17 in f). P < 0.05 was considered as significant. Data in d, h are presented in box-and-whisker plots. Bottom and top of the box, 25th and 75th percentiles; centre line, 50th percentile; whiskers, minimum and maximum data. Two-tailed Student’s t-test was used for statistical test in d, h. ns, no significant differences. sy, synergid cell. Scale bars, 20 μm.
Extended Data Fig. 10 Mutation of active sites of ECS1 and ECS2 endopeptidases leads to polytubey.
a, Mutation of active sites of ECS1 and ECS2 (Extended Data Fig. 2a) led to reduced proteolytic activity. Proteolytic activities of recombinant WT and mutant version of ECS towards cleavage of fluorogenic peptide 3 (Extended Data Fig. 6) were measured respectively. Data represent mean ± s.d. of three independent experiments. b, Mutant version of ECS could not recover the polytubey phenotype of ecs1 ecs2 double mutant. Proportions of polytubey in ecs1 ecs2 double mutants and different transgenic lines were determined at 24 HAP (n = 308 for WT; 302 for ecs1 ecs2; 309 for ECS1; 319 for ECS1D103N D324N-1; 302 for ECS1D103N D324N-2; 309 for ECS2; 321 for ECS2D103N D326N-1; 300 for ECS2D103N D326N-2). Data represent the mean ± s.d. Same letters indicate lack of significant differences according to the Tukey–Kramer multiple comparison test (one-way ANOVA between groups; P = 7.82 × 10−18, F = 50.24). P < 0.05 was considered as significant. c, Representative images showing multiple pollen tubes entrance in different transgenic lines as indicated. Arrows indicate pollen tubes (pt). Scale bars, 100 μm.
Supplementary information
Supplementary Information
This file contains Supplementary Fig. 1 (gel source data) and Supplementary Tables 1-2. Supplementary Table 1 contains a list of primers used in the study and Supplementary Table 2 contains a list of GreenGate modules used for triple reporter line construction.
Rights and permissions
About this article
Cite this article
Yu, X., Zhang, X., Zhao, P. et al. Fertilized egg cells secrete endopeptidases to avoid polytubey. Nature 592, 433–437 (2021). https://doi.org/10.1038/s41586-021-03387-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03387-5
This article is cited by
-
EGG CELL 1 contributes to egg-cell-dependent preferential fertilization in Arabidopsis
Nature Plants (2024)
-
Haploid-Double Haploid Technology for Accelerating Hybrid Development in Maize (Zea mays L.)
Tropical Plant Biology (2023)
-
Two aspartic proteases, BnaAP36s and BnaAP39s, regulate pollen tube guidance in Brassica napus
Molecular Breeding (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.