Abstract
In metazoans, specific tasks are relegated to dedicated organs that are established early in development, occupy discrete locations and typically remain fixed in size. The adult immune system arises from a centralized haematopoietic niche that maintains self-renewing potential1,2, and—upon maturation—becomes distributed throughout the body to monitor environmental perturbations, regulate tissue homeostasis and mediate organism-wide defence. Here we examine how immunity is integrated within adult mouse tissues, and address issues of durability, expansibility and contributions to organ cellularity. Focusing on antiviral T cell immunity, we observed durable maintenance of resident memory T cells up to 450 days after infection. Once established, resident T cells did not require the T cell receptor for survival or retention of a poised, effector-like state. Although resident memory indefinitely dominated most mucosal organs, surgical separation of parabiotic mice revealed a tissue-resident provenance for blood-borne effector memory T cells, and circulating memory slowly made substantial contributions to tissue immunity in some organs. After serial immunizations or cohousing with pet-shop mice, we found that in most tissues, tissue pliancy (the capacity of tissues to vary their proportion of immune cells) enables the accretion of tissue-resident memory, without axiomatic erosion of pre-existing antiviral T cell immunity. Extending these findings, we demonstrate that tissue residence and organ pliancy are generalizable aspects that underlie homeostasis of innate and adaptive immunity. The immune system grows commensurate with microbial experience, reaching up to 25% of visceral organ cellularity. Regardless of the location, many populations of white blood cells adopted a tissue-residency program within nonlymphoid organs. Thus, residence—rather than renewal or recirculation—typifies nonlymphoid immune surveillance, and organs serve as pliant storage reservoirs that can accommodate continuous expansion of the cellular immune system throughout life. Although haematopoiesis restores some elements of the immune system, nonlymphoid organs sustain an accrual of durable tissue-autonomous cellular immunity that results in progressive decentralization of organismal immune homeostasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
ImageJ scripts developed for cell enumeration are available at http://github.com/wijey001/count.
References
Höfer, T., Busch, K., Klapproth, K. & Rodewald, H.-R. Fate mapping and quantitation of hematopoiesis in vivo. Annu. Rev. Immunol. 34, 449–478 (2016).
Sawai, C. M. et al. Hematopoietic stem cells are the major source of multilineage hematopoiesis in adult animals. Immunity 45, 597–609 (2016).
Janeway, C. A., Jr et al. Modes of cell:cell communication in the immune system. J. Immunol. 135, 739s–742s (1985).
Qi, H., Kastenmüller, W. & Germain, R. N. Spatiotemporal basis of innate and adaptive immunity in secondary lymphoid tissue. Annu. Rev. Cell Dev. Biol. 30, 141–167 (2014).
Bromley, S. K. et al. The immunological synapse. Annu. Rev. Immunol. 19, 375–396 (2001).
Mueller, S. N. & Mackay, L. K. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 16, 79–89 (2016).
Szabo, P. A., Miron, M. & Farber, D. L. Location, location, location: tissue resident memory T cells in mice and humans. Sci. Immunol. 4, eaas9673 (2019).
Steinert, E. M. et al. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell 161, 737–749 (2015).
Stark, R. et al. TRM maintenance is regulated by tissue damage via P2RX7. Sci. Immunol. 3, eaau1022 (2018).
Murali-Krishna, K. et al. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science 286, 1377–1381 (1999).
Masopust, D., Vezys, V., Wherry, E. J., Barber, D. L. & Ahmed, R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J. Immunol. 176, 2079–2083 (2006).
Kurd, N. S. et al. Early precursors and molecular determinants of tissue-resident memory CD8+ T lymphocytes revealed by single-cell RNA sequencing. Sci. Immunol. 5, eaaz6894 (2020).
Sallusto, F., Geginat, J. & Lanzavecchia, A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22, 745–763 (2004).
Germain, R. N. & Huang, Y. ILC2s - resident lymphocytes pre-adapted to a specific tissue or migratory effectors that adapt to where they move? Curr. Opin. Immunol. 56, 76–81 (2019).
Klicznik, M. M. et al. Human CD4+CD103+ cutaneous resident memory T cells are found in the circulation of healthy individuals. Sci. Immunol. 4, eaav8995 (2019).
Carbone, F. R. & Gebhardt, T. Should I stay or should I go–reconciling clashing perspectives on CD4+ tissue-resident memory T cells. Sci. Immunol. 4, eaax5595 (2019).
Wu, T. et al. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J. Leukoc. Biol. 95, 215–224 (2014).
Slütter, B. et al. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci. Immunol. 2, eaag2031 (2017).
Stockinger, B., Barthlott, T. & Kassiotis, G. The concept of space and competition in immune regulation. Immunology 111, 241–247 (2004).
Surh, C. D. & Sprent, J. Homeostasis of naive and memory T cells. Immunity 29, 848–862 (2008).
Buck, M. D., Sowell, R. T., Kaech, S. M. & Pearce, E. L. Metabolic instruction of immunity. Cell 169, 570–586 (2017).
Schenkel, J. M. et al. IL-15-independent maintenance of tissue-resident and boosted effector memory CD8 T cells. J. Immunol. 196, 3920–3926 (2016).
Vezys, V. et al. Memory CD8 T-cell compartment grows in size with immunological experience. Nature 457, 196–199 (2009).
Huster, K. M. et al. Cutting edge: memory CD8 T cell compartment grows in size with immunological experience but nevertheless can lose function. J. Immunol. 183, 6898–6902 (2009).
Beura, L. K. et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532, 512–516 (2016).
Gasteiger, G., Fan, X., Dikiy, S., Lee, S. Y. & Rudensky, A. Y. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 350, 981–985 (2015).
Guilliams, M., Thierry, G. R., Bonnardel, J. & Bajenoff, M. Establishment and maintenance of the macrophage niche. Immunity 52, 434–451 (2020).
Schmidt-Rhaesa, A. The Evolution of Organ Systems (Oxford Univ. Press, 2007).
Pabst, O., Herbrand, H., Bernhardt, G. & Förster, R. Elucidating the functional anatomy of secondary lymphoid organs. Curr. Opin. Immunol. 16, 394–399 (2004).
van Furth, R. & Cohn, Z. A. The origin and kinetics of mononuclear phagocytes. J. Exp. Med. 128, 415–435 (1968).
Sallusto, F., Lenig, D., Förster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Weissman, I. L. Stem cells: units of development, units of regeneration, and units in evolution. Cell 100, 157–168 (2000).
Gattinoni, L., Speiser, D. E., Lichterfeld, M. & Bonini, C. T memory stem cells in health and disease. Nat. Med. 23, 18–27 (2017).
Iwasaki, A. Exploiting mucosal immunity for antiviral vaccines. Annu. Rev. Immunol. 34, 575–608 (2016).
Amsen, D., van Gisbergen, K. P. J. M., Hombrink, P. & van Lier, R. A. W. Tissue-resident memory T cells at the center of immunity to solid tumors. Nat. Immunol. 19, 538–546 (2018).
Fonseca, R. et al. Developmental plasticity allows outside-in immune responses by resident memory T cells. Nat. Immunol. 21, 412–421 (2020).
Behr, F. M. et al. Tissue-resident memory CD8+ T cells shape local and systemic secondary T cell responses. Nat. Immunol. 21, 1070–1081 (2020).
Polic, B., Kunkel, D., Scheffold, A. & Rajewsky, K. How αβ T cells deal with induced TCRα ablation. Proc. Natl Acad. Sci. USA 98, 8744–8749 (2001).
Ruzankina, Y. et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 1, 113–126 (2007).
Tucker, C. G. et al. Adoptive T cell therapy with IL-12-preconditioned low-avidity T cells prevents exhaustion and results in enhanced T cell activation, enhanced tumor clearance, and decreased risk for autoimmunity. J. Immunol. 205, 1449–1460 (2020).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Anderson, K. G. et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat. Protoc. 9, 209–222 (2014).
Klose, C. S. N. et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549, 282–286 (2017).
Guilliams, M. et al. Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity 45, 669–684 (2016).
Jiang, X. et al. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature 483, 227–231 (2012).
Acknowledgements
We thank members of the laboratories of D.M. and V.V. for helpful discussions; C. Klose and D. Artis for advice in identifying innate lymphoid cells; University of Minnesota Flow Cytometry Resource; University Imaging Centers (J. Mitchell and T. Pengo); and the Biosafety Level 3 Program. This study was supported by National Institutes of Health (NIH) grants R01 AI084913, R01 AI146032 (D.M.), F30 DK114942 and T32 AI007313 (S.W.) and the Howard Hughes Medical Institute Faculty Scholars program (D.M.).
Author information
Authors and Affiliations
Contributions
S.W., L.K.B., M.J.P., J.M.S., O.A.A., R.R., E.M.S. and P.C.R. performed the experiments; S.W., V.V. and D.M. designed the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Evan Newell and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Compartmentalized decay of uterine T cells concomitant with morphological changes in tissue architecture over time.
a, Representative immunofluorescence of uterine tissue. b, The frequency of P14 memory CD8+ T cells in uterine compartments was assessed by quantitative immunofluorescent microscopy at day 60 (n = 6 mice) and day 200 (n = 7 mice) after LCMV infection in one experiment. c, Representative immunofluorescence images of mouse uterine tissue at various ages, demonstrating endometrial vacuolations in older mice. d, e, Representative immunofluorescence images of mouse salivary gland at various time points demonstrating emergence of salivary gland tertiary lymphoid organs in older mice (d) and expression of peripheral node addressin (PNAd) (e). Morphology representative of n > 12 mice, PNAd staining representative of n = 5 mice (c–e). Scale bar, 100 μm (a), 500 μm (c, 70 weeks in d), 200 μm (10 and 35 weeks in d, e). Statistical significance was determined by two-tailed Mann–Whitney U test (b). *P = 0.0221, **P = 0.0023 (endometrium) or **P = 0.0082 (perimetrium). Data are mean ± s.e.m.
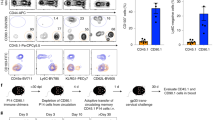
Extended Data Fig. 2 Selective TCR ablation using Tracfl/fl mice reveals TCR-independent homeostasis of TRM cells.
a, Experimental model. Thy1.1−CD45.2+ Tracfl/fl mice and Thy1.1+CD45.2+ wild-type B6 mice were infected with LCMV. After 30 days, 107 lymphocytes—isolated from secondary lymphoid organs—were transferred into naive CD45.1+ B6 mice, which were subsequently infected with LCMV. Forty days after infection, CD45.1+ mice were treated with tamoxifen to selectively ablate TCR from transferred Thy1.1−CD45.2+ Tracfl/fl secondary memory T cells. b, c, LCMV-specific secondary memory T cells in peripheral blood are shown 40 days after LCMV infection (before tamoxifen treatment) (b). Data pooled from 3 independent experiments for a total of n = 8 mice (c). d, e, Selective TCR ablation of Tracfl/fl secondary memory CD8+ T cells, as measured by ex vivo peptide stimulation assay. Sixty days after tamoxifen treatment of CD45.1+ B6 recipient mice, splenocytes were isolated and stimulated in vitro with gp33–41 peptide. Cytokine production by TCR− Tracfl/fl memory CD8+ T cells and TCR+ wild-type memory CD8+ T cells from spleen is shown, and reflects n = 6 mice. f, Frequency of cells that lack TCRβ expression on Tracfl/fl memory CD8+ T cells. Data pooled from 4 independent experiments for a total of n = 8–10 mice (n varies by tissue). g, Representative flow cytometry, depicting expression of tissue-resident markers on small-intestine epithelial memory CD8+ T cells 60 days after tamoxifen treatment. h, Frequency of CD69+ memory CD8+ T cells in the spleen for wild-type and TCRβ− Tracfl/fl populations. Data pooled from four independent experiments, for a total of n = 10 mice. Statistical significance was determined by two-tailed Wilcoxon matched-pairs signed-rank test (e, h). *P = 0.0313. Data are mean ± s.e.m.
Extended Data Fig. 3 In vitro activation of Tracfl/fl naive T cells generates primary TRM cells that are maintained in the absence of constitutive TCR signalling.
a, Experimental model. Lymphocytes were isolated from secondary lymphoid organs of CD45.2+ Tracfl/fl mice and wild-type Thy1.1+ B6 mice, and enriched for naive CD8+ T cells via magnetic bead enrichment. T cells were activated in vitro for 3 days with anti-CD3ε and rB7-1, and 107 cells were co-transferred into naive CD45.1+ B6 mice. Thirty days later, recipient mice were treated with tamoxifen. b, Thirty days after tamoxifen treatment, transferred CD8+ T cells were evaluated for CD44 expression, as compared to endogenous CD8+ T cells, shown via representative flow cytometry of CD8+ T cells isolated from blood. c, Expression of TCRβ was evaluated for Tracfl/fl and wild-type CD8+ T cells, as shown via representative flow cytometry of peripheral blood. d, The ratio of Tracfl/fl to wild-type CD8+ T cells was quantified 30 days after tamoxifen treatment in various tissues, normalized to values from blood, and was not significantly different from 1:1. Data show n = 4 biologically independent mice from 1 experiment. Statistical significance was determined by two-tailed one-sample Wilcoxon test, using 0 as a hypothetical mean. Data are box plots showing median, IQR and extremes.
Extended Data Fig. 4 CD69 does not unequivocally distinguish long-lived TRM cells in the lung.
a, b, Representative flow cytometry (a) and graph (b), demonstrating the degree of disequilibrium among CD69+ extravascular memory P14 CD8+ T cells in tissues of separated parabiotic mice (n = 8–10), 260 days after LCMV infection from 1 experiment. Top panels in a are gated on extravascular memory CD8+ P14 T cells. Data are mean ± s.e.m.
Extended Data Fig. 5 Ex-TRM cells comprise a substantial fraction of blood-borne memory.
a, b, Longitudinal graphs depicting the frequency of host-derived memory P14 CD8+ T cells (a) or the frequency of ex-TRM cells of P14 CD8+ T cells, as calculated (b) in the peripheral blood of separated parabiotic mice from two independent experiments (n = 17). Data are mean ± s.e.m.; in b, coloured dotted lines reflect s.e.m. c, d, More than 200 days after separation of congenically distinct parabiotic P14-immune chimeric mice (n = 17), host- and donor-derived P14 CD8+ T cells were evaluated for expression of markers of antigen experience, tissue-trafficking and differentiation potential (d). Gating strategy for P14 CD8+ T cells in separated parabiotic mice shown in c is generally representative of the flow cytometry panels in Figs. 1, 2, Extended Data Figs. 2–4, 6.
Extended Data Fig. 6 The glycoform of CD43 recognized by 1B11 is expressed on CD8+ TRM cells.
a, b, Representative flow cytometry (a) and quantification (b) of CD43–1B11 antibody staining on memory P14 CD8+ T cells in nonlymphoid tissues of mice (n = 9) 200 days after infection with LCMV. In a, naive CD8+ T cells isolated from peripheral blood (in red) serve as basis for comparison. Data are mean ± s.e.m.
Extended Data Fig. 7 Pre-existing memory T cells retain functional potency after heterologous prime–boost immunization.
a, Sixty days after infection with LCMV, P14-immune chimeric mice were subjected to a heterologous prime–boost regimen. The ex vivo functionality of memory P14 CD8+ T cells in various tissues was compared, and found to be not significantly different (P > 0.05) between n = 4 or 5 mice (n varies by tissue) receiving heterologous prime–boost and n = 5 age-matched control mice, from one of two independent experiments with similar results. Statistical significance was determined by two-tailed Mann–Whitney U test. Data are mean ± s.e.m.
Extended Data Fig. 8 Lung or skin memory CD8+ T cells are preserved after microbial experience.
a–d, P14 CD8+ T cells were transferred into naive mice, which were intranasally infected with PR8–gp33 influenza virus and, 30 days later, mice were cohoused for 45 days with mice obtained from pet shops (a). P14 CD8+ T cells from spleen (b), extravascular lung (c) and bronchoalveolar lavage (BAL) fluid (d) of cohoused mice (n = 8) were enumerated and compared to infection-matched mice housed in SPF conditions (n = 8) from 1 experiment. e–g, OT-1 CD8+ T cells were transferred into naive mice, which were intravenously infected with VSV–OVA; 30 days later, mice were cohoused for 60 days with mice obtained from pet shops (e). OT-1 CD8+ T cells from spleen (f) and epidermal skin (g) of cohoused mice (n = 6) were enumerated and compared to infection-matched mice housed in SPF conditions (n = 7) from 1 experiment. Statistical significance was determined by two-tailed Mann–Whitney U test. **P = 0.0047 (b); **P = 0.0012 (f). Data are box plots showing median, IQR and extremes.
Extended Data Fig. 9 Both CD4+ and CD8+ memory T cell populations are expansible.
a, b, CD45+ cells increase in tissues after cohousing (Fig. 3). Here we examined relative frequencies of memory T cells. C57Bl/6 SPF laboratory mice were cohoused for >60 days with mice obtained from pet shops. Age-matched, conventionally housed SPF mice served as controls. The frequency of CD4+ memory T cells (a) and CD8+ memory T cells (b) as a proportion of CD45+ immune cells is depicted in various tissues in both groups of mice. Memory T cells were defined as CD44+PD1−. mLN, mesenteric lymph node. Data are pooled from 2–4 independent experiments for a total of n = 4–14 mice (n varies by tissue) per group. Data are mean ± s.e.m.
Extended Data Fig. 10 Tissue residence typifies immune surveillance for many leukocyte populations.
a, Model depicting the cohousing of CD45.1+ and CD45.2+ C57Bl/6 SPF laboratory mice for >60 days with mice obtained from pet shops, followed by parabiosis of laboratory mice for 28–32 days. b, Between 28 and 32 days after parabiosis, the equilibration of leukocyte populations in peripheral blood was evaluated in n = 8–14 mice. c–h, Between 28 and 32 days after parabiosis, the tissue disequilibrium of innate lymphoid cells (c, n = 3–12 mice), natural killer cells (d, n = 5–14 mice), monocytes and macrophages (e, n = 4–12 mice), CD44+PD1− memory T cells (f, n = 7–14 mice), granulocytes (g, n = 4–12 mice) and B cells (h, n = 2–14 mice) was evaluated. Data are pooled from four independent experiments and n varies dependent on tissue and population of interest (as not all cell populations were abundantly detected in each tissue or each experiment). AM, alveolar macrophages; IM, interstitial macrophages; mes LN, mesenteric lymph node. Data are mean ± s.e.m.
Supplementary information
Source data
Rights and permissions
About this article
Cite this article
Wijeyesinghe, S., Beura, L.K., Pierson, M.J. et al. Expansible residence decentralizes immune homeostasis. Nature 592, 457–462 (2021). https://doi.org/10.1038/s41586-021-03351-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03351-3
This article is cited by
-
Joint-specific memory, resident memory T cells and the rolling window of opportunity in arthritis
Nature Reviews Rheumatology (2024)
-
Prostaglandin E2 controls the metabolic adaptation of T cells to the intestinal microenvironment
Nature Communications (2024)
-
Stem-like exhausted and memory CD8+ T cells in cancer
Nature Reviews Cancer (2023)
-
Metabolic programs of T cell tissue residency empower tumour immunity
Nature (2023)
-
CD4+ T cell memory
Nature Immunology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.