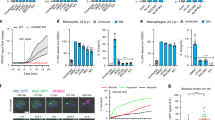
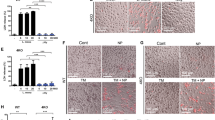
Abstract
Plasma membrane rupture (PMR) is the final cataclysmic event in lytic cell death. PMR releases intracellular molecules known as damage-associated molecular patterns (DAMPs) that propagate the inflammatory response1,2,3. The underlying mechanism of PMR, however, is unknown. Here we show that the cell-surface NINJ1 protein4,5,6,7,8, which contains two transmembrane regions, has an essential role in the induction of PMR. A forward-genetic screen of randomly mutagenized mice linked NINJ1 to PMR. Ninj1−/− macrophages exhibited impaired PMR in response to diverse inducers of pyroptotic, necrotic and apoptotic cell death, and were unable to release numerous intracellular proteins including HMGB1 (a known DAMP) and LDH (a standard measure of PMR). Ninj1–/– macrophages died, but with a distinctive and persistent ballooned morphology, attributable to defective disintegration of bubble-like herniations. Ninj1–/– mice were more susceptible than wild-type mice to infection with Citrobacter rodentium, which suggests a role for PMR in anti-bacterial host defence. Mechanistically, NINJ1 used an evolutionarily conserved extracellular domain for oligomerization and subsequent PMR. The discovery of NINJ1 as a mediator of PMR overturns the long-held idea that cell death-related PMR is a passive event.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
RNA-sequencing data are available through the Gene Expression Omnibus (GEO) database at accession number GSE156395. Datasets from UniProt database (https://www.uniprot.org/) including the mouse and contaminant subsets as well as the following accession numbers: Q70131, Q92982, P70617, F1PMB0, Q2TA30, R4GJU8, H9G4V3, Q66JI7, A0A0R4IDX9, Q9NZG7 and Q9JL89 were used. Other datasets generated during and/or analysed during the current study are available from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Fink, S. L. & Cookson, B. T. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 73, 1907–1916 (2005).
Gaidt, M. M. & Hornung, V. Pore formation by GSDMD is the effector mechanism of pyroptosis. EMBO J. 35, 2167–2169 (2016).
Kayagaki, N. & Dixit, V. M. Rescue from a fiery death: a therapeutic endeavor. Science 366, 688–689 (2019).
Araki, T. & Milbrandt, J. Ninjurin, a novel adhesion molecule, is induced by nerve injury and promotes axonal growth. Neuron 17, 353–361 (1996).
Araki, T., Zimonjic, D. B., Popescu, N. C. & Milbrandt, J. Mechanism of homophilic binding mediated by ninjurin, a novel widely expressed adhesion molecule. J. Biol. Chem. 272, 21373–21380 (1997).
Ahn, B. J. et al. The N-terminal ectodomain of Ninjurin1 liberated by MMP9 has chemotactic activity. Biochem. Biophys. Res. Commun. 428, 438–444 (2012).
Lee, H. J. et al. Ninjurin1 mediates macrophage-induced programmed cell death during early ocular development. Cell Death Differ. 16, 1395–1407 (2009).
Yang, H. J. et al. Ninjurin 1 has two opposing functions in tumorigenesis in a p53-dependent manner. Proc. Natl Acad. Sci. USA 114, 11500–11505 (2017).
Cookson, B. T. & Brennan, M. A. Pro-inflammatory programmed cell death. Trends Microbiol. 9, 113–114 (2001).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Ding, J. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016).
Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016).
Fink, S. L. & Cookson, B. T. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell. Microbiol. 8, 1812–1825 (2006).
Ruan, J., Xia, S., Liu, X., Lieberman, J. & Wu, H. Cryo-EM structure of the gasdermin A3 membrane pore. Nature 557, 62–67 (2018).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011).
de Vasconcelos, N. M., Van Opdenbosch, N., Van Gorp, H., Parthoens, E. & Lamkanfi, M. Single-cell analysis of pyroptosis dynamics reveals conserved GSDMD-mediated subcellular events that precede plasma membrane rupture. Cell Death Differ. 26, 146–161 (2019).
DiPeso, L., Ji, D. X., Vance, R. E. & Price, J. V. Cell death and cell lysis are separable events during pyroptosis. Cell Death Discov. 3, 17070 (2017).
Andersson, U. & Tracey, K. J. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 29, 139–162 (2011).
Štros, M. HMGB proteins: interactions with DNA and chromatin. Biochim. Biophys. Acta 1799, 101–113 (2010).
Silva, M. T. Secondary necrosis: the natural outcome of the complete apoptotic program. FEBS Lett. 584, 4491–4499 (2010).
Ashkenazi, A., Fairbrother, W. J., Leverson, J. D. & Souers, A. J. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat. Rev. Drug Discov. 16, 273–284 (2017).
Dijkstra, K., Hofmeijer, J., van Gils, S. A. & van Putten, M. J. A biophysical model for cytotoxic cell swelling. J. Neurosci. 36, 11881–11890 (2016).
Sun, L. et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 (2012).
Grootjans, S., Vanden Berghe, T. & Vandenabeele, P. Initiation and execution mechanisms of necroptosis: an overview. Cell Death Differ. 24, 1184–1195 (2017).
Drin, G. & Antonny, B. Amphipathic helices and membrane curvature. FEBS Lett. 584, 1840–1847 (2010).
Peter, B. J. et al. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303, 495–499 (2004).
Auluck, P. K., Caraveo, G. & Lindquist, S. α-Synuclein: membrane interactions and toxicity in Parkinson’s disease. Annu. Rev. Cell Dev. Biol. 26, 211–233 (2010).
Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 415, 389–395 (2002).
Hatzakis, N. S. et al. How curved membranes recruit amphipathic helices and protein anchoring motifs. Nat. Chem. Biol. 5, 835–841 (2009).
Nelms, K. A. & Goodnow, C. C. Genome-wide ENU mutagenesis to reveal immune regulators. Immunity 15, 409–418 (2001).
Kayagaki, N. et al. IRF2 transcriptionally induces GSDMD expression for pyroptosis. Sci. Signal. 12, eaax4917 (2019).
Andrews, T. D. et al. Massively parallel sequencing of the mouse exome to accurately identify rare, induced mutations: an immediate source for thousands of new mouse models. Open Biol. 2, 120061 (2012).
Murphy, J. M. et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39, 443–453 (2013).
Modzelewski, A. J. et al. Efficient mouse genome engineering by CRISPR-EZ technology. Nat. Protocols 13, 1253–1274 (2018).
Aglietti, R. A. et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc. Natl Acad. Sci. USA 113, 7858–7863 (2016).
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Contrepois, K. et al. Cross-platform comparison of untargeted and targeted lipidomics approaches on aging mouse plasma. Sci. Rep. 8, 17747 (2018).
Bakalarski, C. E. et al. The impact of peptide abundance and dynamic range on stable-isotope-based quantitative proteomic analyses. J. Proteome Res. 7, 4756–4765 (2008).
Wang, G. G. et al. Quantitative production of macrophages or neutrophils ex vivo using conditional Hoxb8. Nat. Methods 3, 287–293 (2006).
Wittig, I., Braun, H. P. & Schägger, H. Blue native PAGE. Nat. Protocols 1, 418–428 (2006).
Waterhouse, A. et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46 (W1), W296–W303 (2018).
Ashkenazy, H., Erez, E., Martz, E., Pupko, T. & Ben-Tal, N. ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 38, W529–W533 (2010).
Ashkenazy, H. et al. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 44, W344–W350 (2016).
UniProt Consortium. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 47 (D1), D506–D515 (2019).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011).
Robert, X. & Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42, W320-4 (2014).
Acknowledgements
We thank the staff of the Australian Phenomics Facility, Genentech Transgenic Technology, imaging cores, and antibody engineering group, S. Wu, S. Gierke, F. Gallardo-Chang, Z. Altshuler and W. Fairbrother for technical expertise and discussion, K. Newton for manuscript editing, and W. Alexander for Mlkl–/– mice. Figures 2b, k, 3h were created with biorender.com.
Author information
Authors and Affiliations
Contributions
N.K., O.S.K., B.L.L., I.B.S., K.O., J.P., Z.M., V.C., T.D.A, L.X.M. and L.A.M. designed and performed experiments, Q.L. and W.S. performed secretome and lipidomics analysis, D.Y., J.K., M.X., J.Z., W.P.L. and B.S.M. performed in vivo studies, M.R.-G. generated the Ninj1–/– mice, G.U. performed the liposomal cargo release assay, O.S.K. and R.R. provided computational analysis, O.S.K., I.B.S., M.S. and J.D.W. performed microscopic analysis, and C.C.G., E.M.B. and V.M.D. contributed to experimental design. N.K. wrote the paper with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
N.K., O.S.K., B.L.L., I.B.S., K.O., Q.L., W.S., D.Y., J.K., M.X., J.Z., W.P.L., B.S.M., G.U., J.P., M.R.G., Z.M., R.R., M.S., J.D.B. and V.M.D. are all employees of Genentech Inc.
Additional information
Peer review information Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Homozygosity of the Ninj1 point mutation correlates with low responsiveness to LPS.
a, Ninj1 genotypes and screen phenotypes of mice derived from IGL03767. Identification numbers of screened mice are shown. G, generation. b, Nineteen SNV genotypes mutated in the pedigree and their phenotypes. c, Wild-type and IGL03767 Ninj1 genes. Exon 2 coding sequence is in uppercase and SNV mutation is in bold and highlighted with an asterisk. Grey boxes represent exons.
Extended Data Fig. 2 NINJ1 is essential for pyroptosis-related PMR.
a, Immunoblot of NINJ1 and GSDMD in BMDM lysates. b, Left, release of DD-150 in live-cell imaging analysis of iMACs after LPS electroporation over a 16-h time course. Right, immunoblot of NINJ1 in Ninj1–/– iMACs reconstituted with NINJ1. Data are means (circles) ± s.d. (shaded area) of three individual replicates. c, Immunoblot of GSDMD, GSDMD-NT and NINJ1 in supernatant and extract from nigericin-stimulated primed BMDMs. Actin is from a separate blot. d, LDH or IL-18 release from BMDMs stimulated as in Fig. 2d. e, IL-6 or TNF production from BMDMs stimulated with Pam3CSK4 (TLR2) or extracellular LPS (TLR4). f, Lipid composition of primed BMDMs. Lipids profiled are diacylglycerol (DAG), dihydroceramide (DCER), hexosylceramide (HCER), lactosylceramide (LCER), lysophosphatidylcholine (LPC), lysophosphatidylethanolamine (LPE), phosphatidylcholine (PC), phosphatidylethanolamine (PE), sphingomyelin (SM), triacylglycerol (TAG), cholesteryl ester (CE), and ceramide (CER). g, RNA-seq of primed BMDMs. h, Time-lapse imaging analysis of mitochondrial membrane potential as measured by tetramethylrhodamine methyl ester perchlorate (TMRM) in primed BMDMs following LPS electroporation. Data are means (circles) ± s.d. (shaded area) of three individual replicates. i, Single-cell time-lapse imaging analysis of TMRM and DD-150 release in primed wild-type BMDMs after LPS electroporation. Time point ‘0’ corresponds to the point of maximal TMRM decline for each cell. j, Silver staining of proteins in culture supernatant of nigericin-stimulated primed BMDMs. k, Kaplan–Meier survival plots for mice challenged with 54 mg kg−1 LPS. P values were calculated by a two-sided Gehan–Breslow–Wilcoxon test. Unless otherwise specified, data are means (bars) of at least three individual replicates (circles). n = 2 per genotype. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 3 NINJ1 has a global role in PMR induction related to pyroptosis, apoptosis and necrosis.
a, d, g, Release of HMGB1 from BMDMs stimulated with cisplatin, venetoclax, oligomycin (a, d), or TNF plus zVAD (g). b, Viability (left) and LDH release (right) of venetoclax-stimulated BMDMs. c, Expression of indicated gasdermin transcripts in RNA-seq analysis of BMDMs. FPKM, fragments per kilobase of transcript per million mapped reads. e, Bright-field images of BMDMs cultured with oligomycin. Scale bars, 25 μm. f, Silver staining of proteins in culture supernatant of BMDMs stimulated with TNF and zVAD. Unless otherwise specified, data are means (bars) of at least three individual replicates (circles). n = 2 per genotype. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 4 Phylogenetic tree and amino acid sequence alignment of NINJ1 and NINJ2.
a, Immunoblot of Flag–NINJ1 from Fig. 4a; representative of two independent experiments. b, LDH release from Flag–NINJ1 transfected HEK293T cells. Data are means (bars) of at least three individual replicates (circles) c, Cytotoxicity of non-tagged human and mouse NINJ1, NINJ2, and dNINJ-A, B, C in HEK293T cells. Data are means (bars) of at least four individual replicates (circles). d, Phylogenetic tree of NINJ1 and NINJ2. Numbers represent standardized distance scores (number of amino acid substitutions per length of the alignment). e, Multiple alignment of NINJ1 and NINJ2 amino acid sequences with the extracellular α-helix domain predicted by JPred highlighted in blue. f, Multiple alignment of NINJ1 amino acid sequences with the extracellular α-helix domain predicted by JPred. g, Circular dichroism spectra of NINJ1 α-helix region peptide that shows characteristic α-helix pattern (two dips at 208 nm and 222 nm). h, Immunoblot of Flag–NINJ1 from Fig. 4b; representative of two independent experiments. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 5 Biochemical analysis of NINJ1.
a, Top, NINJ1 domain structure. Middle, cytotoxicity of Flag-tagged wild-type NINJ1 or NINJ1 with proline point mutants in HEK293T cells. Numbers indicate positions of amino acid residues that were replaced by proline. Cytotoxicity (killing score) was normalized against wild-type NINJ1 control. Bottom, immunoblot of Flag–NINJ1. Data are means (bars) of at least three individual replicates (circles). b, Left, cytotoxicity of Flag-tagged wild-type NINJ1 or NINJ1 mutants in HEK293T cells. Right, immunoblot of Flag–NINJ1. Data are means (bars) of at least four individual replicates (circles). c, Left, release of DD-150 in live-cell imaging of Ninj1–/– iMACs reconstituted with NINJ1 after LPS electroporation. Right, immunoblot of iMACs with anti-NINJ1 polyclonal antibody. Data are means (circles) ± s.d. (shaded area) of three individual replicates. d, e, Immunoblot of NINJ1 in primed BMDMDs stimulated with LPS electroporation or nigericin (d), or in non-primed BMDMDs cultured with indicated stimuli (e). f, Phos-tag SDS–PAGE analysis of LPS electroporation- or nigericin-stimulated primed BMDMs with or without staurosporine pre-treatment. S6, ribosomal protein S6. Results in d–f are representative of two independent experiments. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 6 Subcellular localization of NINJ1.
Immunofluorescence microscopy of NINJ1 and indicated markers in primed BMDMs. Scale bars, 25 μm. Micrographs are representative of two independent experiments.
Extended Data Fig. 7 Characterization of NINJ1 extracellular α-helix domain.
a, Computational model of the NINJ1 extracellular domain. Grey, hydrophobic residues; blue, negatively charged residues; light blue, polar residues; red, positively charged residues. b, Immunoblot of Flag–NINJ1 in HEK293T cells from Fig. 4e; representative of two independent experiments. c, Liposome cargo release by the NINJ1 α-helix region peptide, its sequence scrambled variant, or Melittin (α-helical bee venom peptide). Data are means (bars) of four individual replicates (circles). P value was calculated using a two-tailed unpaired Student’s t-test. d, Release of DD-150 in live-cell imaging of BMDMs after stimulation by nigericin in the presence of 20 μg ml−1 of indicated monoclonal antibodies. Data are means (circles) ± s.d. (shaded area) of four individual replicates. mAb, monoclonal antibody. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 8 Characterization of Gsdmd–/–Gsdme–/– BMDMs and anti-NINJ1 monoclonal antibody.
a, Immunoblot of GSDME in BMDMs. GSDME is from a separate blot. b, Immunoblots with anti-mouse NINJ1 monoclonal antibody clone 25 in HEK293T cells transfected with the indicated Flag-tagged constructs. Results in a and b are representative of two independent experiments. For gel source data, see Supplementary Fig. 1.
Supplementary information
Supplementary Figure 1
Uncropped gel source data.
Video 1
Ninj1–/– BMDMs die with persistent ballooned morphology in response to nigericin. Time-lapse imaging of primed BMDMs following nigericin stimulation (every 5 min for a total of 800 min).
Video 2
Ninj1–/– BMDMs die with persistent ballooned morphology in response to LPS. Time-lapse imaging of primed BMDMs following LPS transfection (every 5 min for a total of 800 min).
Video 3
Lattice light-sheet microscopy of Ninj1–/– BMDMs cells. Time-lapse lattice light-sheet microscopy imaging of primed Ninj1–/– BMDMs following nigericin stimulation (every 30 sec for a total of 60 min). Green, CellMask™ Plasma membrane Stain.
Video 4
Venetoclax-stimulated Ninj1–/– BMDMs retain prominent bubble morphology. Time-lapse imaging of venetoclax-stimulated BMDMs (every 3 min after 290 min of stimulation for 430 min).
Rights and permissions
About this article
Cite this article
Kayagaki, N., Kornfeld, O.S., Lee, B.L. et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 591, 131–136 (2021). https://doi.org/10.1038/s41586-021-03218-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03218-7
This article is cited by
-
Multiomics characterization of pyroptosis in the tumor microenvironment and therapeutic relevance in metastatic melanoma
BMC Medicine (2024)
-
Structural and functional insights of NINJ1 in plasma membrane rupture during cell death
Molecular Biomedicine (2024)
-
NINJ1 mediates inflammatory cell death, PANoptosis, and lethality during infection conditions and heat stress
Nature Communications (2024)
-
The role of the NLRP3 inflammasome and pyroptosis in cardiovascular diseases
Nature Reviews Cardiology (2024)
-
Mechanistic insights from inflammasome structures
Nature Reviews Immunology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.