Abstract
One of the long-standing challenges in experimental physics is the observation of room-temperature superconductivity1,2. Recently, high-temperature conventional superconductivity in hydrogen-rich materials has been reported in several systems under high pressure3,4,5. An important discovery leading to room-temperature superconductivity is the pressure-driven disproportionation of hydrogen sulfide (H2S) to H3S, with a confirmed transition temperature of 203 kelvin at 155 gigapascals3,6. Both H2S and CH4 readily mix with hydrogen to form guest–host structures at lower pressures7, and are of comparable size at 4 gigapascals. By introducing methane at low pressures into the H2S + H2 precursor mixture for H3S, molecular exchange is allowed within a large assemblage of van der Waals solids that are hydrogen-rich with H2 inclusions; these guest–host structures become the building blocks of superconducting compounds at extreme conditions. Here we report superconductivity in a photochemically transformed carbonaceous sulfur hydride system, starting from elemental precursors, with a maximum superconducting transition temperature of 287.7 ± 1.2 kelvin (about 15 degrees Celsius) achieved at 267 ± 10 gigapascals. The superconducting state is observed over a broad pressure range in the diamond anvil cell, from 140 to 275 gigapascals, with a sharp upturn in transition temperature above 220 gigapascals. Superconductivity is established by the observation of zero resistance, a magnetic susceptibility of up to 190 gigapascals, and reduction of the transition temperature under an external magnetic field of up to 9 tesla, with an upper critical magnetic field of about 62 tesla according to the Ginzburg–Landau model at zero temperature. The light, quantum nature of hydrogen limits the structural and stoichiometric determination of the system by X-ray scattering techniques, but Raman spectroscopy is used to probe the chemical and structural transformations before metallization. The introduction of chemical tuning within our ternary system could enable the preservation of the properties of room-temperature superconductivity at lower pressures.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information files, and from the corresponding author upon reasonable request.
Change history
30 August 2021
Editor's Note: The editors of Nature have been alerted to undeclared access restrictions relating to the data behind this paper. We are working with the authors to correct the data availability statement.
31 January 2022
Editor’s Note: The authors have made available raw data pertaining to the magnetic susceptibility measurements in the paper. These can be accessed at https://arxiv.org/abs/2111.15017.
15 February 2022
Editor’s Note: The editors of Nature have been alerted to concerns regarding the manner in which the data in this paper have been processed and interpreted. Nature is working with the authors to investigate these concerns and establish what (if any) impact they will have on the paper’s results and conclusions. In the meantime, readers are advised to use caution when using results reported therein.
20 November 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41586-020-2955-8
26 September 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41586-022-05294-9
References
Onnes, H. K. The resistance of pure mercury at helium temperatures. Commun. Phys. Lab. Univ. Leiden 12, 1 (1911).
Ginzburg, V. L. Nobel Lecture: on superconductivity and superfluidity (what I have and have not managed to do) as well as on the “physical minimum” at the beginning of the XXI century. Rev. Mod. Phys. 76, 981–998 (2004).
Drozdov, A. P., Eremets, M. I., Troyan, I. A., Ksenofontov, V. & Shylin, S. I. Conventional superconductivity at 203 kelvin at high pressures in the sulfur hydride system. Nature 525, 73–76 (2015).
Drozdov, A. P. et al. Superconductivity at 250 K in lanthanum hydride under high pressures. Nature 569, 528–531 (2019).
Somayazulu, M. et al. Evidence for superconductivity above 260 K in lanthanum superhydride at megabar pressures. Phys. Rev. Lett. 122, 027001 (2019).
Duan, D. et al. Pressure-induced metallization of dense (H2S)2H2 with high-Tc superconductivity. Sci. Rep. 4, 6968 (2014).
Strobel, T. A., Ganesh, P., Somayazulu, M., Kent, P. R. C. & Hemley, R. J. Novel cooperative interactions and structural ordering in H2S–H2. Phys. Rev. Lett. 107, 255503 (2011).
Bi, T., Zarifi, N., Terpstra, T. & Zurek, E. The search for superconductivity in high pressure hydrides. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering https://doi.org/10.1016/B978-0-12-409547-2.11435-0 (Elsevier, 2019).
Sun, Y., Lv, J., Xie, Y., Liu, H. & Ma, Y. Route to a superconducting phase above room temperature in electron-doped hydride compounds under high pressure. Phys. Rev. Lett. 123, 097001 (2019).
Pickard, C. J., Errea, I. & Eremets, M. I. Superconducting hydrides under pressure. Annu. Rev. Condens. Matter Phys. 11, 57–76 (2020).
Shimizu, K., Suhara, K., Ikumo, M., Eremets, M. I. & Amaya, K. Superconductivity in oxygen. Nature 393, 767–769 (1998).
Struzhkin, V. V., Hemley, R. J., Mao, H. & Timofeev, Y. A. Superconductivity at 10–17 K in compressed sulphur. Nature 390, 382–384 (1997).
Dias, R. P. et al. Superconductivity in highly disordered dense carbon disulfide. Proc. Natl Acad. Sci. USA 110, 11720–11724 (2013).
Kim, D. Y., Scheicher, R. H., Mao, H., Kang, T. W. & Ahuja, R. General trend for pressurized superconducting hydrogen-dense materials. Proc. Natl Acad. Sci. USA 107, 2793–2796 (2010).
Tanaka, K., Tse, J. S. & Liu, H. Electron-phonon coupling mechanisms for hydrogen-rich metals at high pressure. Phys. Rev. B 96, 100502 (2017).
Ashcroft, N. W. Metallic hydrogen: a high-temperature superconductor? Phys. Rev. Lett. 21, 1748–1749 (1968).
Dias, R. P. & Silvera, I. F. Observation of the Wigner–Huntington transition to metallic hydrogen. Science 355, 715–718 (2017).
Eremets, M. I., Drozdov, A. P., Kong, P. P. & Wang, H. Semimetallic molecular hydrogen at pressure above 350 GPa. Nat. Phys. 15, 1246–1249 (2019).
Zaghoo, M., Salamat, A. & Silvera, I. F. Evidence of a first-order phase transition to metallic hydrogen. Phys. Rev. B 93, 155128 (2016).
Wang, H., Tse, J. S., Tanaka, K., Iitaka, T. & Ma, Y. Superconductive sodalite-like clathrate calcium hydride at high pressures. Proc. Natl Acad. Sci. USA 109, 6463–6466 (2012).
Liu, H., Naumov, I. I., Hoffmann, R., Ashcroft, N. W. & Hemley, R. J. Potential high-Tc superconducting lanthanum and yttrium hydrides at high pressure. Proc. Natl Acad. Sci. USA 114, 6990–6995 (2017).
Peng, F. et al. Hydrogen clathrate structures in rare earth hydrides at high pressures: possible route to room-temperature superconductivity. Phys. Rev. Lett. 119, 107001 (2017).
Errea, I. et al. High-pressure hydrogen sulfide from first principles: a strongly anharmonic phonon-mediated superconductor. Phys. Rev. Lett. 114, 157004 (2015).
Nagamatsu, J., Nakagawa, N., Muranaka, T., Zenitani, Y. & Akimitsu, J. Superconductivity at 39 K in magnesium diboride. Nature 410, 63–64 (2001).
Cui, W. et al. Route to high-Tc superconductivity via CH4-intercalated H3S hydride perovskites. Phys. Rev. B 101, 134504 (2020).
Sun, Y. et al. Computational discovery of a dynamically stable cubic SH3-like high-temperature superconductor at 100 GPa via CH4 intercalation. Phys. Rev. B 101, 174102 (2020).
Einaga, M. et al. Crystal structure of the superconducting phase of sulfur hydride. Nat. Phys. 12, 835–838 (2016).
Little, W. A. Possibility of synthesizing an organic superconductor. Phys. Rev. 134, A1416–A1424 (1964).
Ginzburg, V. L. On surface superconductivity. Phys. Lett. 13, 101–102 (1964).
Akahama, Y. & Kawamura, H. Pressure calibration of diamond anvil Raman gauge to 310 GPa. J. Appl. Phys. 100, 043516 (2006).
Hsieh, S. et al. Imaging stress and magnetism at high pressures using a nanoscale quantum sensor. Science 366, 1349–1354 (2019).
Lesik, M. et al. Magnetic measurements on micrometer-sized samples under high pressure using designed NV centers. Science 366, 1359–1362 (2019).
Yip, K. Y. et al. Measuring magnetic field texture in correlated electron systems under extreme conditions. Science 366, 1355–1359 (2019).
Mozaffari, S. et al. Superconducting phase diagram of H3S under high magnetic fields. Nat. Commun. 10, 2522 (2019).
Eckert, B. & Schumacher, R., Jodl, H. J. & Foggi, P. Pressure and photo-induced phase transitions in sulphur investigated by Raman spectroscopy. High Press. Res. 17, 113–146 (2000).
Somayazulu, M. S., Finger, L. W., Hemley, R. J. & Mao, H. K. High-pressure compounds in methane-hydrogen mixtures. Science 271, 1400–1402 (1996).
Kearney, J. S. C. et al. Pressure-tuneable visible-range band gap in the ionic spinel tin nitride. Angew. Chem. Int. Ed. 57, 11623–11628 (2018).
Spiekermann, G. et al. Persistent octahedral coordination in amorphous GeO2 up to 100 GPa by Kβ″ X-ray emission spectroscopy. Phys. Rev. X 9, 011025 (2019).
Dias, R. P., Noked, O. & Silvera, I. F. Quantum phase transition in solid hydrogen at high pressure. Phys. Rev. B 100, 184112 (2019).
Dias, R. P., Noked, O. & Silvera, I. F. New phases and dissociation-recombination of hydrogen deuteride to 3.4 Mbar. Phys. Rev. Lett. 116, 145501 (2016).
Frank, R. B. in Mössbauer Effect Methodology 151–180 (Springer, 1976).
Debessai, M., Hamlin, J. J. & Schilling, J. S. Comparison of the pressure dependences of Tc in the trivalent d-electron superconductors Sc, Y, La, and Lu up to megabar pressures. Phys. Rev. B 78, 064519 (2008).
Hohenberg, P. & Kohn, W. Inhomogeneous electron gas. Phys. Rev. 136, B864–B871 (1964).
Kohn, W. & Sham, L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133–A1138 (1965).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Lejaeghere, K. et al. Reproducibility in density functional theory calculations of solids. Science 351, aad3000 (2016).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Froyen, S. & Cohen, M. Structural properties of NaCl and KCl under pressure. J. Phys. C 19, 2623–2632 (1986).
Dacosta, P. G., Nielsen, O. H. & Kunc, K. Stress theorem in the determination of static equilibrium by the density functional method. J. Phys. C 19, 3163–3172 (1986).
Vanderbilt, D. Absence of large compressive stress on Si(111). Phys. Rev. Lett. 59, 1456–1459 (1987).
Francis, G. P. & Payne, M. C. Finite basis set corrections to total energy pseudopotential calculations. J. Phys. Condens. Matter 2, 4395–4404 (1990).
Hazen, R. M., Mao, H. K., Finger, L. W. & Bell, P. M. Structure and compression of crystalline methane at high pressure and room temperature. Appl. Phys. Lett. 37, 288–289 (1980).
Zhou, D. et al. Elastic properties of single crystal hydrogen sulfide: a Brillouin scattering study under high pressure-temperature. J. Appl. Phys. 124, 125901 (2018).
Pratesi, G., Ulivi, L., Barocchi, F., Loubeyre, P. & Le Toullec, R. Hyperacoustic velocity of fluid hydrogen at high pressure. J. Phys. Condens. Matter 9, 10059–10064 (1997).
Darkrim, F. & Levesque, D. Monte Carlo simulations of hydrogen adsorption in single-walled carbon nanotubes. J. Chem. Phys. 109, 4981–4984 (1998).
Somayazulu, M. S., Finger, L. W., Hemley, R. J. & Mao, H. K. High-pressure compounds in methane-hydrogen mixtures. Science 271, 1400–1402 (1996).
Pace, E. J. et al. Properties and phase diagram of (H2S)2H2. Phys. Rev. B 101, 174511 (2020).
Das, A. et al. The H2S dimer is hydrogen-bonded: direct confirmation from microwave spectroscopy. Angew. Chem. Int. Ed. 57, 15199–15203 (2018).
Bernal, J. D. & Fowler, R. H. A theory of water and ionic solution, with particular reference to hydrogen and hydroxyl ions. J. Chem. Phys. 1, 515–548 (1933).
Acknowledgements
We thank N. Meyers for technical support during the initial stage of the project. Also, we thank L. Koelbl for discussions on the manuscript. Preparation of diamond surfaces was performed in part at the University of Rochester Integrated Nanosystems Center. This research was supported by NSF (grant number DMR-1809649) and the DOE Stockpile Stewardship Academic Alliance Program (grant number DE-NA0003898). This work supported by the US Department of Energy, Office of Science, Fusion Energy Sciences under award number DE-SC0020340. A.S. and K.V.L. are supported by DE-SC0020303.
Author information
Authors and Affiliations
Contributions
E.S., N.D.-G. and R.M. performed the Raman and electrical conductivity measurements and contributed to the writing of the paper; K.V. participated in the Raman measurements and analysis of the Raman data. H.V. analysed the low-temperature magnetic-field-dependent electrical conductivity measurements and contributed to the writing of the paper. M.D. provided technical support during the initial stage of the electrical conductivity measurements, performed magnetic susceptibility measurements and contributed to the writing of the paper. R.P.D. conceived the project and performed electrical conductivity and magnetic susceptibility experiments. K.V.L. and A.S. analysed the data and the chemistry protocol. K.V.L., A.S. and R.P.D. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1038/s41586-022-05294-9
Extended data figures and tables
Extended Data Fig. 1 Vibrational frequencies of the C–S–H sample.
Pressure versus Raman shift for low-frequency modes compared to pure sulfur modes. Grey solid markers are from the sample; the green, red and blue crosses represent different phases of pure sulfur.
Extended Data Fig. 2 Raman spectra of the C–S–H sample.
Spectral deconvolution of Raman spectra of the C–S–H compound used to find the peak positions reported in Fig. 3b. a.u., arbitrary units.
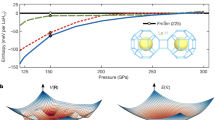
Extended Data Fig. 3 Superconducting properties of the C–S–H system.
a, Coherence length versus temperature. b, Temperature dependence of the penetration depth. The penetration depth can be determined to be λ(0) > 3.8 nm as \(\lambda (0)={\varphi }_{0}/[2\sqrt{2}{\rm{\pi }}{H}_{{\rm{c}}}(0)\xi (0)]\,\) with Hc(0) = 61.8 T and ξ(0) = 2.3 nm; ϕ0 is the magnetic flux quantum. c, GL parameter, κ = λ(T)/ξ(T) at \(T=0\); \(\kappa > 1/\sqrt{2}\) indicates type II superconductivity. In our experiment κ > 1.1, so the sample can be identified as a type II superconductor. d, Variation of the superconducting bandgap Δ versus temperature, \(\varDelta (T)/\varDelta (0)=1.76{k}_{{\rm{B}}}\,{T}_{{\rm{c}}}\sqrt{1-(T/{T}_{{\rm{c}}})}\) (kB, Boltzmann constant).
Extended Data Fig. 4 Superconducting transition at 272 GPa.
Temperature-dependent quasi-four-point electric resistance measurement of the C–S–H sample at 272 GPa, showing the superconducting transitions at ~280 K. Tc was determined from the onset of superconductivity (see arrow). A superconducting step is observed at the transition midpoint.
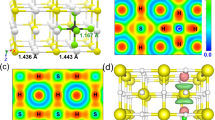
Extended Data Fig. 5 A DFT-optimized structure for (H2S)2H2 at 4 GPa.
View along the (00−1) (left) and (–100) (right) planes of a calculated snapshot of (H2S)2H2. S–H distances between 1.4 Å and 2.8 Å are shown as dashed lines to indicate potential hydrogen bonds.
Extended Data Fig. 6 A DFT-optimized structure for (H2S)(CH4)H2 (variant 2) at 4 GPa.
View along the (00−1) (left) and (−100) (right) planes of a calculated snapshot of a variant (number 2) of (H2S)(CH4)H2. S–H distances between 1.4 Å and 2.8 Å are shown as dashed lines to indicate potential hydrogen bonds.
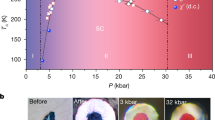
Extended Data Fig. 7 Additional data on superconducting transition and pressure measurements.
a, Representative spectra of the diamond Raman edge used for pressure determination. b, Plot of resistance versus temperature, showing that the width of the superconducting drop is less than 1 K. Inset, zoom-in of the resistance near 0 Ω. c, Temperature dependence of the resistance of C–S–H at different pressures (different samples). d, Temperature dependence of the measured a.c. susceptibility of C–S–H at 182 GPa. The inset shows raw data at 138 GPa.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-4.
Video 1
Photochemical synthesis of C-S-H system. The video shows the sample chamber inside of a diamond anvil cell, looking axially through the top diamond. The sample originally exists of carbon and sulfur, shown as a black powder, sitting inside a hydrogen-rich environment. The sample is at 4 GPa and is illuminated by 10 mW of 532 nm of laser light for several minutes to initiate and take to completion the photochemical reaction to produce a CSH Van der Waals solid. This sample can be seen to be grown with a single crystal-like motif.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Snider, E., Dasenbrock-Gammon, N., McBride, R. et al. RETRACTED ARTICLE: Room-temperature superconductivity in a carbonaceous sulfur hydride. Nature 586, 373–377 (2020). https://doi.org/10.1038/s41586-020-2801-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2801-z
This article is cited by
-
Full-bandwidth anisotropic Migdal-Eliashberg theory and its application to superhydrides
Communications Physics (2024)
-
The characterization of superconductivity under high pressure
Nature Materials (2024)
-
Superconductivity scandal: the inside story of deception in a rising star’s physics lab
Nature (2024)
-
The future of academic integrity in the age of artificial intelligence
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
-
Hopes raised for room-temperature superconductivity, but doubts remain
Nature (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.