Abstract
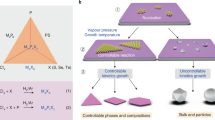
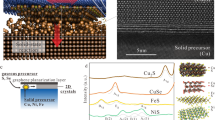
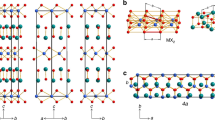
Although two-dimensional (2D) atomic layers, such as transition-metal chalcogenides, have been widely synthesized using techniques such as exfoliation1,2,3 and vapour-phase growth4,5, it is still challenging to obtain phase-controlled 2D structures6,7,8. Here we demonstrate an effective synthesis strategy via the progressive transformation of non-van der Waals (non-vdW) solids to 2D vdW transition-metal chalcogenide layers with identified 2H (trigonal prismatic)/1T (octahedral) phases. The transformation, achieved by exposing non-vdW solids to chalcogen vapours, can be controlled using the enthalpies and vapour pressures of the reaction products. Heteroatom-substituted (such as yttrium and phosphorus) transition-metal chalcogenides can also be synthesized in this way, thus enabling a generic synthesis approach to engineering phase-selected 2D transition-metal chalcogenide structures with good stability at high temperatures (up to 1,373 kelvin) and achieving high-throughput production of monolayers. We anticipate that these 2D transition-metal chalcogenides will have broad applications for electronics, catalysis and energy storage.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors on reasonable request.
References
Coleman, J. N. et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 331, 568–571 (2011).
Lin, Z. et al. Solution-processable 2D semiconductors for high-performance large-area electronics. Nature 562, 254–258 (2018).
Huang, Y. et al. Reliable exfoliation of large-area high-quality flakes of graphene and other two-dimensional materials. ACS Nano 9, 10612–10620 (2015).
Zhou, J. et al. A library of atomically thin metal chalcogenides. Nature 556, 355–359 (2018).
Chen, X. et al. CVD-grown monolayer MoS2 in bioabsorbable electronics and biosensors. Nat. Commun. 9, 1690–1701 (2018).
Voiry, D. et al. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat. Mater. 12, 850–855 (2013).
Zhu, J. et al. Argon plasma induced phase transition in monolayer MoS2. J. Am. Chem. Soc. 139, 10216–10219 (2017).
Eda, G. et al. Photoluminescence from chemically exfoliated MoS2. Nano Lett. 11, 5111–5116 (2011).
Zhang, J. et al. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 1, 985–992 (2018).
Kundu, D., Adams, B. D., Ort, V. D., Vajargah, S. H. & Nazar, L. F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 1, 16119–16126 (2016).
Zhang, C. et al. High capacity silicon anodes enabled by MXene viscous aqueous ink. Nat. Commun. 10, 849–857 (2019).
Yan, M. et al. Water-lubricated intercalation in V2O5·nH2O for high-capacity and high-rate aqueous rechargeable zinc batteries. Adv. Mater. 30, 1703725–1703730 (2018).
Wang, Y. et al. In situ exfoliated, N-doped, and edge-rich ultrathin layered double hydroxides nanosheets for oxygen evolution reaction. Adv. Funct. Mater. 28, 1703363–1703368 (2018).
Liu, J., Qian, X. & Fu, L. Crystal field effect induced topological crystalline insulators in monolayer IV–VI semiconductors. Nano Lett. 15, 2657–2661 (2015).
Balan, A. P. et al. Exfoliation of a non-van der Waals material from iron ore hematite. Nat. Nanotechnol. 13, 602–609 (2018).
Naguib, M. et al. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 23, 4248–4253 (2011).
Barsoum, M. W. MAX Phases: Properties of Machinable Ternary Carbides and Nitrides (Wiley, 2013).
Sokol, M., Natu, V., Kota, S. & Barsoum, M. W. On the chemical diversity of the MAX phases. Trends Chem. 1, 210–223 (2019).
Xuan, J. et al. Organic-base-driven intercalation and delamination for the production of functionalized titanium carbide nanosheets with superior photothermal therapeutic performance. Angew. Chem. Int. Ed. 55, 14569–14574 (2016).
Ghidiu, M., Lukatskaya, M. R., Zhao, M. Q., Gogotsi, Y. & Barsoum, M. W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 516, 78–81 (2014).
Lukatskaya, M. R. et al. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 341, 1502–1505 (2013).
Li, M. et al. Element replacement approach by reaction with Lewis acidic molten salts to synthesize nanolaminated MAX phases and MXenes. J. Am. Chem. Soc. 141, 4730–4737 (2019).
Barin, I. Thermochemical Data of Pure Substances 3rd edn (VCH, 1995).
Yaws, C. L. The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals 2nd edn (Elsevier, 2015).
Winterbone, D. E. Advanced Thermodynamics for Engineers (Butterworth-Heinemann, 1997).
Naguib, M., Mochalin, V. N., Barsoum, M. W. & Gogotsi, Y. 25th anniversary article: MXenes: a new family of two-dimensional materials. Adv. Mater. 26, 992–1005 (2014).
Anasori, B., Lukatskaya, M. R. & Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2, 16098–16114 (2017).
Anasori, B. et al. Two-dimensional, ordered, double transition metals carbides (MXenes). ACS Nano 9, 9507–9516 (2015).
Meshkian, R. et al. W-based atomic laminates and their 2D derivative W1.33C MXene with vacancy ordering. Adv. Mater. 30, 1706409–1706416 (2018).
Naguib, M. et al. Two-dimensional transition metal carbides. ACS Nano 6, 1322–1331 (2012).
Deepak, F. L. et al. Fullerene-like (IF) NbxMo1-xS2 nanoparticles. J. Am. Chem. Soc. 129, 12549–12562 (2007).
Yang, S. Z. et al. Rhenium-doped and stabilized MoS2 atomic layers with basal-plane catalytic activity. Adv. Mater. 30, 1803477–1803483 (2018).
Liu, Q. et al. Stable metallic 1T-WS2 nanoribbons intercalated with ammonia ions: the correlation between structure and electrical/optical properties. Adv. Mater. 27, 4837–4844 (2015).
Radhakrishnan, S. et al. An insight into the phase transformation of WS2 upon fluorination. Adv. Mater. 30, 1803366–1803375 (2018).
Lin, Y. C., Dumcencon, D. O., Huang, Y. S. & Suenaga, K. Atomic mechanism of the semiconducting-to-metallic phase transition in single-layered MoS2. Nat. Nanotechnol. 9, 391–396 (2014).
Sun, Z. M. Progress in research and development on MAX phases: a family of layered ternary compounds. Int. Mater. Rev. 56, 143–166 (2011).
Barsoum, M. W. The MN+1AXN phases: a new class of solids: thermodynamically stable nanolaminates. Prog. Solid State Chem. 28, 201–281 (2000).
Eklund, P., Beckers, M., Jansson, U., Hogberg, H. & Hultman, L. The Mn+1AXn phases: materials science and thin-film processing. Thin Solid Films 518, 1851–1878 (2010).
Magnuson, M. & Mattesini, M. Chemical bonding and electronic-structure in MAX phases as viewed by X-ray spectroscopy and density functional theory. Thin Solid Films 621, 108–130 (2017).
Naguib, M. et al. New two-dimensional niobium and vanadium carbides as promising materials for Li-ion batteries. J. Am. Chem. Soc. 135, 15966–15969 (2013).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (grant numbers 51622203 and 51572007), the Youth 1000-Talent Program of China and the 111 Project (grant number B17002). X.Z. also thanks Shenzhen Basic Research Projects (grant number JCYJ20170407155608882), the China Postdoctoral Science Foundation (grant number 2018M631458) and the Development and Reform Commission of Shenzhen Municipality for the development of the “Low-Dimensional Materials and Devices” Discipline, Guangdong Innovative and Entrepreneurial Research Team Program (grant number 2017ZT07C341). We thank the Shanghai Synchrotron Radiation Facility and Beijing Synchrotron Radiation Facility for support. We thank X. Chen, Q. Zhang, W. Zhou and M. Li for help with the TEM analysis; S. Chen and Y. Lin for help with the EXAFS analysis; and L. Ma for suggestions.
Author information
Authors and Affiliations
Contributions
S.Y., S.L. and P.M.A. supervised the project. S.Y. and Z.D. designed and carried out all of the experiments. S.Z. and X.Z. carried out the density functional theory calculations. S.W. and B.L. performed the scanning electron microscope, TEM and X-ray diffraction measurements. B.L. carried out the X-ray photoelectron spectroscopy and Raman analysis. L.S. contributed to the XANES and EXAFS measurements. Z.D. performed the atomic force microscopy. Z.D. and Y.G. designed the film electrodes and carried out the electrical and electrocatalytic measurements. All authors discussed the results and assisted during manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Per Eklund, Wei Sun Leong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains the Supplementary Information (sections 1-6), which includes Supplementary Materials and Methods, Supplementary Figures 1-73, Supplementary Tables 1-9, Supplementary Text and Supplementary References–see contents page for details.
Rights and permissions
About this article
Cite this article
Du, Z., Yang, S., Li, S. et al. Conversion of non-van der Waals solids to 2D transition-metal chalcogenides. Nature 577, 492–496 (2020). https://doi.org/10.1038/s41586-019-1904-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1904-x
This article is cited by
-
Epitaxial substitution of metal iodides for low-temperature growth of two-dimensional metal chalcogenides
Nature Nanotechnology (2023)
-
Emergence of superconductivity in 2D Cu0.65NbS2 nanosheets via copper ion hopping
Science China Materials (2023)
-
Applications of MXenes in human-like sensors and actuators
Nano Research (2023)
-
Quantum Dots Compete at the Acme of MXene Family for the Optimal Catalysis
Nano-Micro Letters (2022)
-
Advances and challenges in two-dimensional materials for oxygen evolution
Nano Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.