Abstract
Dietary habits and vascular risk factors promote both Alzheimer’s disease and cognitive impairment caused by vascular factors1,2,3. Furthermore, accumulation of hyperphosphorylated tau, a microtubule-associated protein and a hallmark of Alzheimer’s pathology4, is also linked to vascular cognitive impairment5,6. In mice, a salt-rich diet leads to cognitive dysfunction associated with a nitric oxide deficit in cerebral endothelial cells and cerebral hypoperfusion7. Here we report that dietary salt induces hyperphosphorylation of tau followed by cognitive dysfunction in mice, and that these effects are prevented by restoring endothelial nitric oxide production. The nitric oxide deficiency reduces neuronal calpain nitrosylation and results in enzyme activation, which, in turn, leads to tau phosphorylation by activating cyclin-dependent kinase 5. Salt-induced cognitive impairment is not observed in tau-null mice or in mice treated with anti-tau antibodies, despite persistent cerebral hypoperfusion and neurovascular dysfunction. These findings identify a causal link between dietary salt, endothelial dysfunction and tau pathology, independent of haemodynamic insufficiency. Avoidance of excessive salt intake and maintenance of vascular health may help to stave off the vascular and neurodegenerative pathologies that underlie dementia in the elderly.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Source data include final quantifications from in vivo animal work.
Change history
14 January 2020
An Amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Scarmeas, N., Anastasiou, C. A. & Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 17, 1006–1015 (2018).
van de Rest, O., Berendsen, A. A., Haveman-Nies, A. & de Groot, L. C. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv. Nutr. 6, 154–168 (2015).
Kendig, M. D. & Morris, M. J. Reviewing the effects of dietary salt on cognition: mechanisms and future directions. Asia Pac. J. Clin. Nutr. 28, 6–14 (2019).
De Strooper, B. & Karran, E. The cellular phase of Alzheimer’s disease. Cell 164, 603–615 (2016).
Nation, D. A. et al. Pulse pressure in relation to tau-mediated neurodegeneration, cerebral amyloidosis, and progression to dementia in very old adults. JAMA Neurol. 72, 546–553 (2015).
Kim, H. J. et al. Assessment of extent and role of tau in subcortical vascular cognitive impairment using 18F-AV1451 positron emission tomography imaging. JAMA Neurol. 75, 999–1007 (2018).
Faraco, G. et al. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat. Neurosci. 21, 240–249 (2018).
Fiocco, A. J. et al. Sodium intake and physical activity impact cognitive maintenance in older adults: the NuAge Study. Neurobiol. Aging 33, 829.e821–829.e28, (2012).
Gardener, H., Rundek, T., Wright, C. B., Elkind, M. S. & Sacco, R. L. Dietary sodium and risk of stroke in the Northern Manhattan study. Stroke 43, 1200–1205 (2012).
Blumenthal, J. A. et al. Lifestyle and neurocognition in older adults with cognitive impairments: a randomized trial. Neurology 92, e212–e223 (2019).
Heye, A. K. et al. Blood pressure and sodium: association with MRI markers in cerebral small vessel disease. J. Cereb. Blood Flow Metab. 36, 264–274 (2016).
Iadecola, C. The pathobiology of vascular dementia. Neuron 80, 844–866 (2013).
Sweeney, M. D. et al. Vascular dysfunction—the disregarded partner of Alzheimer’s disease. Alzheimers Dement. 15, 158–167 (2019).
Shi, Y. et al. Cerebral blood flow in small vessel disease: a systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 36, 1653–1667 (2016).
Marshall, R. S. et al. Recovery of brain function during induced cerebral hypoperfusion. Brain 124, 1208–1217 (2001).
Wang, Y. & Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 17, 5–21 (2016).
Zhao, Y. et al. Sodium intake regulates glucose homeostasis through the PPARδ/adiponectin-mediated SGLT2 pathway. Cell Metab. 23, 699–711 (2016).
Min, S. W. et al. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat. Med. 21, 1154–1162 (2015).
Iadecola, C. et al. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat. Neurosci. 2, 157–161 (1999).
Faraco, G. et al. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J. Clin. Invest. 126, 4674–4689 (2016).
Arendt, T., Stieler, J. T. & Holzer, M. Tau and tauopathies. Brain Res. Bull. 126, 238–292 (2016).
Lee, M. S. et al. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405, 360–364 (2000).
Patrick, G. N. et al. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402, 615–622 (1999).
Austin, S. A. & Katusic, Z. S. Loss of endothelial nitric oxide synthase promotes p25 generation and tau phosphorylation in a murine model of Alzheimer’s disease. Circ. Res. 119, 1128–1134 (2016).
Bibb, J. A. et al. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature 402, 669–671 (1999).
Shukla, V. et al. A truncated peptide from p35, a Cdk5 activator, prevents Alzheimer’s disease phenotypes in model mice. FASEB J. 27, 174–186 (2013).
Kimura, T. et al. Isomerase Pin1 stimulates dephosphorylation of tau protein at cyclin-dependent kinase (Cdk5)-dependent Alzheimer phosphorylation sites. J. Biol. Chem. 288, 7968–7977 (2013).
Ono, Y., Saido, T. C. & Sorimachi, H. Calpain research for drug discovery: challenges and potential. Nat. Rev. Drug Discov. 15, 854–876 (2016).
Etwebi, Z., Landesberg, G., Preston, K., Eguchi, S. & Scalia, R. Mechanistic role of the calcium-dependent protease calpain in the endothelial dysfunction induced by MPO (myeloperoxidase). Hypertension 71, 761–770 (2018).
Qu, J. et al. S-Nitrosylation activates Cdk5 and contributes to synaptic spine loss induced by β-amyloid peptide. Proc. Natl Acad. Sci. USA 108, 14330–14335 (2011).
Iadecola, C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96, 17–42 (2017).
Yanamandra, K. et al. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron 80, 402–414 (2013).
Powles, J. et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 3, e003733 (2013).
Hochrainer, K. et al. The ubiquitin ligase HERC3 attenuates NF-κB-dependent transcription independently of its enzymatic activity by delivering the RelA subunit for degradation. Nucleic Acids Res. 43, 9889–9904 (2015).
Shukla, V. et al. TFP5, a peptide inhibitor of aberrant and hyperactive Cdk5/p25, attenuates pathological phenotypes and restores synaptic function in CK-p25Tg mice. J. Alzheimers Dis. 56, 335–349 (2017).
Faraco, G. et al. Circulating endothelin-1 alters critical mechanisms regulating cerebral microcirculation. Hypertension 62, 759–766 (2013).
Kober, F. et al. High-resolution myocardial perfusion mapping in small animals in vivo by spin-labeling gradient-echo imaging. Magn. Reson. Med. 51, 62–67 (2004).
Petry, F. R. et al. Specificity of anti-tau antibodies when analyzing mice models of Alzheimer’s disease: problems and solutions. PLoS One 9, e94251 (2014).
Faraco, G. et al. Hypertension enhances Aβ-induced neurovascular dysfunction, promotes β-secretase activity, and leads to amyloidogenic processing of APP. J. Cereb. Blood Flow Metab. 36, 241–252 (2016).
Voit, A. et al. Reducing sarcolipin expression mitigates Duchenne muscular dystrophy and associated cardiomyopathy in mice. Nat. Commun. 8, 1068 (2017).
Liu, W. et al. Metabolic stress-induced cardiomyopathy is caused by mitochondrial dysfunction due to attenuated Erk5 signaling. Nat. Commun. 8, 494 (2017).
Forrester, M. T., Foster, M. W., Benhar, M. & Stamler, J. S. Detection of protein S-nitrosylation with the biotin-switch technique. Free Radic. Biol. Med. 46, 119–126 (2009).
Cohen, S. J. & Stackman, R. W. Jr Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav. Brain Res. 285, 105–117 (2015).
Grayson, B. et al. Assessment of disease-related cognitive impairments using the novel object recognition (NOR) task in rodents. Behav. Brain Res. 285, 176–193 (2015).
O’Leary, T. P. & Brown, R. E. Optimization of apparatus design and behavioral measures for the assessment of visuo-spatial learning and memory of mice on the Barnes maze. Learn. Mem. 20, 85–96 (2013).
Acknowledgements
We thank P. Davies for providing the RZ3, MC1 and PHF1 antibodies and Y. Li for sharing the Quickplex SQ 120 system (Meso Scale Diagnostics LLC). This study was supported by National Institutes of Health grants R37-NS089323 (C.I.) and 1R01-NS095441 (C.I.), by a grant from the Cure Alzheimer’s Fund (G.F. and C.I.) and by a Scientist Development Grant from the American Heart Association (G.F.). Support from the Feil Family Foundation is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
G.F. performed western blotting experiments, behavioural tests and cerebrovascular studies, and analysed data. K.H. performed experiments on CDK5 and GSK3β activity and analysed data. S.G.S. performed western blotting experiments, behavioural tests and immunohistochemistry. S.S. and M.M.S. performed experiments on the effects of hypertension on tau. A.M. performed immunohistochemistry experiments. H.J. and D.M.H. provided the HJ8.8 antibody. J.A. supervised the molecular aspects of the study and edited the manuscript. G.F. and C.I. designed and supervised the entire study and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
D.M.H. is listed as an inventor on a patent licensed by Washington University to C2N Diagnostics and subsequently AbbVie on the therapeutic use of anti-tau antibodies; co-founded and is on the scientific advisory board of C2N Diagnostics; and is on the scientific advisory board of Denali, Genentech, and Proclara. C.I. is on the scientific advisory board of Broadview Ventures.
Additional information
Peer review information Nature thanks Nikolaos Scarmeas, Berislav Zlokovic and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 HSD (4 or 8%) induced tau phosphorylation: brain localization, sex differences and time course.
a, HSD (8% NaCl) increases tau phosphorylation on Ser396 (PHF13) and on Ser199Ser202 in the hippocampus (HIPP) but not in the neocortex (CTX) (HIPP: PHF13, ND/HSD n = 4/5, *P = 0.0016; Ser199Ser202, ND/HSD n = 4/5, *P = 0.0337; two-tailed unpaired t-test versus ND), whereas acetylation of tau on Lys280 (K280) is not affected. MC1 immunoreactivity increases in both neocortex and hippocampus in HSD-fed mice but reaches statistical significance only in neocortex (MC1, ND/HSD n = 4/5, *P = 0.0321 versus ND, two-tailed unpaired t-test). b, Tau phosphorylation on Ser199Ser202 and Ser202Thr205 is abolished after treatment of brain samples with lambda phosphatase. c, HSD increases AT8 immunoreactivity (left graph) in neocortex and hippocampus of female mice, but RZ3 (middle) increases only in neocortex (AT8, cortex: ND/HSD n = 8/10, *P = 0.0159; hippocampus: ND/HSD n = 10/9, *P = 0.0151; RZ3, cortex: ND/HSD n = 10/8, *P = 0.0117; two-tailed unpaired t-test for HSD versus ND). Right, HSD induces a deficit in NOR in female mice (ND/HSD n = 8/9, *P = 0.0017 versus ND, two-tailed unpaired t-test). d, HSD increases AT8 immunoreactivity in neuronal cell bodies of the somatosensory cortex (scale bars, 100 μm (main images); 10 µm (insets)) and MC1 immunoreactivity in neuronal bodies of the pyriform cortex (scale bars, 500 μm (main images); 100 µm (insets)). Representative images from ND- and HSD-fed mice (n = 5 per group). e, Thioflavin S staining is not present in mice fed an HSD, indicating absence of neurofibrillary tangles, which can be observed in rTg4510 mice (scale bars, 500 μm (main images); 100 µm (inset)). Representative images from n = 5 ND- and HSD-fed mice and n = 3 rTg4510 mice. f, HSD (4%) increases AT8 immunoreactivity in the neocortex but not in the hippocampus (AT8, cortex: ND/HSD n = 5/5, *P = 0.0148 versus ND, two-tailed unpaired t-test). RZ3 was not increased in both regions. g, Time course of the increase in AT8 and RZ3 induced by HSD in the hippocampus. AT8 levels are increased after 4 weeks of HSD. RZ3 levels are increased after 4, 12, 24 and 36 weeks of HSD (AT8, 4 weeks: ND/HSD n = 4/5, *P = 0.0386; 12 weeks: ND/HSD n = 9/9, *P < 0.0001; RZ3, 4 weeks: ND/HSD n = 4/5, *P = 0.0041; RZ3, 12 weeks: ND/HSD n = 9/9, *P = 0.0011; 24 weeks: ND/HSD n = 7/10, *P = 0.0017; 36 weeks: ND/HSD n = 5/4, *P = 0.0188; two-tailed unpaired t-test for HSD versus ND). For gel source data see Supplementary Fig. 1. Data are expressed as mean ± s.e.m.
Extended Data Fig. 2 Effect of HSD on neurons, astrocytes, microglia/macrophages, pericytes and white-matter integrity.
a, HSD (NaCl 8%) does not affect neurons (NEUN), astrocytes (GFAP), microglia/macrophages (IBA-1) (cortex: microglia, ND/HSD n = 5/5, P = 0.0570; hippocampus: ND/HSD n = 5/5, P = 0.0556; two-tailed unpaired t-test for HSD versus ND) or pericytes (CD13) in both the pyriform cortex and the hippocampus (scale bars, 500 μm (except where specified)). b, No evidence of neuronal cell death is observed in HSD-fed mice by Fluoro-Jade B or TUNEL staining (scale bar, 500 μm). +DNase indicates positive control for TUNEL staining. Representative images from ND- and HSD-fed mice (n = 5 per group). c, Klüver–Barrera stain shows no white-matter damage in the corpus callosum of HSD-fed mice (scale bar, 100 μm). Representative images from ND- and HSD-fed mice (n = 4 per group). Data are expressed as mean ± s.e.m.
Extended Data Fig. 3 Aβ levels in HSD-fed mice and correlation of behavioural deficits with p-tau, as well as p-tau in hypertension, HSD-treated tg2576 mice and hypothermia.
a, HSD (8% NaCl) does not alter the distance travelled before finding the escape hole in the Barnes maze (primary distance, ND/HSD n = 13/13, diet: *P = 0.0462, time: *P < 0.0001, two-way repeated measures ANOVA and Bonferroni’s test; primary distance day 5: ND/HSD n = 13/13, P = 0.0670 versus ND, two-tailed unpaired t-test) or the number of errors made (primary errors, ND/HSD n = 13/13, diet: P = 0.110, time: *P = 0.0004, two-way repeated measures ANOVA and Bonferroni’s test; primary errors day 5: P = 0.1226 versus ND, two-tailed unpaired t-test). b, RZ3 levels in the cortex correlate with cognitive performance on the NOR test. No correlation was found between hippocampal RZ3 levels and cognitive performance on either the Barnes maze or the NOR test. RZ3 cortex: Barnes maze r = 0.2828, *P = 0.0133, n = 76; NOR r = −0.2806, *P = 0.0170, n = 72; RZ3 hippocampus: Barnes maze r = 0.1739, P = 0.1470, n = 71; NOR r = −0.1746, P = 0.1577, n = 67, Pearson’s correlation coefficient). c, HSD does not increase soluble or insoluble Aβ38, Aβ40 or Aβ42 in the neocortex. Aβ38, soluble ND/HSD n = 11/9, insoluble ND/HSD n = 7/6; Aβ40, soluble ND/HSD n = 11/14, insoluble ND/HSD n = 7/6; Aβ42, soluble ND/HSD n = 9/9, insoluble ND/HSD n = 7/6. d, Delivery of angiotensin II (ANGII; 600 ng kg−1 min−1, subcutaneously (s.c.)) via osmotic minipumps over 6 weeks increases systolic blood pressure (SBP) and induces cognitive deficits (SBP: Veh/ANGII n = 10/10, treatment: *P < 0.0001, time: *P < 0.0001, repeated measures two-way ANOVA and Bonferroni’s test; NOR: 2 weeks Veh/ANGII n = 12/12, 4 weeks Veh/ANGII n = 10/11, 6 weeks Veh/ANGII n = 7/7, treatment: *P < 0.0021, time: *P = 0.0208, two-way ANOVA and Bonferroni’s test). e, Administration of angiotensin II increases AT8 and RZ3 immunoreactivity in the neocortex but not the hippocampus (cortex, AT8 6 weeks: Veh/ANGII n = 4/4, *P = 0.0324; RZ3 6 weeks: Veh/ANGII n = 5/5, *P = 0.0262; hippocampus, AT8 6 weeks: Veh/ANGII n = 5/5, P = 0.4056; RZ3 6 weeks: Veh/ANGII n = 5/5, P = 0.0556, two-tailed unpaired t-test versus vehicle). f, HSD increases AT8 and RZ3 levels in both the neocortex and the hippocampus of 6-month-old Tg2576 mice (cortex, AT8: *P < 0.0001; hippocampus, AT8: *P = 0.0153; cortex, RZ3: *P < 0.0001; hippocampus, RZ3: *P = 0.0239; two-tailed unpaired t-test for HSD versus ND). g, Hypothermia induces massive AT8 phosphorylation (cortex: AT8 n = 4/5, *P = 0.0159; hippocampus: AT8 n = 4/5, *P = 0.0159) and increases MC1 (cortex: MC1 n = 4/5, *P = 0.0317; hippocampus: MC1 n = 4/5, *P = 0.0159) and RZ3 (cortex: RZ3 n = 4/5, *P = 0.0201; hippocampus: RZ3 n = 4/5, *P = 0.0453). Unpaired two-tailed t-test for hypothermia (HYPO) versus normal temperature (NT). h, Unlike HSD (Fig. 1G), hypothermia does not shift tau from soluble to more insoluble fractions. For gel source data see Supplementary Fig. 1. Data are expressed as mean ± s.e.m.
Extended Data Fig. 4 Effect of l-arginine on p-tau and calpain expression, as well as p-tau in eNOS−/− mice, calpain and CDK5 localization, pDARPP-32 with HSD, and IL-17 levels.
a, Administration of l-arginine (10 g l−1 in drinking water), starting at week 8 of HSD and continuing through week 12, suppresses RZ3 levels in the neocortex but not in the hippocampus (cortex: RZ3, ND/HSD n = 10/10, *P < 0.0001; hippocampus: RZ3, ND/HSD n = 10/10, *P = 0.0005, two-tailed unpaired t-test versus normal diet with vehicle). b, l-Arginine does not affect the increase in serum IL-17 induced by HSD (Veh, ND/HSD n = 9/11, *P = 0.0002 versus ND Veh; l-arg, ND/HSD n = 9/8, *P < 0.0001 versus ND l-arg, two-tailed unpaired t-test). c, AT8 and RZ3 levels are elevated in the neocortex and hippocampus of eNOS−/− mice on ND (AT8: cortex, ND/HSD n = 5/4, *P = 0.0029; hippocampus, ND/HSD n = 5/4, *P = 0.0078; RZ3: cortex, ND/HSD n = 5/4, *P = 0.0003; hippocampus, ND/HSD n = 5/4, *P = 0.0128, two-tailed unpaired t-test versus wild-type mice). d, HSD does not increase tau phosphorylation in eNOS−/− mice (RZ3: hippocampus, ND/HSD n = 7/8, *P = 0.0224 versus ND, two-tailed unpaired t-test). e, Calpain 2 immunoreactivity is present in neuronal cell bodies of the somatosensory and piriform cortex (scale bars, 500 μm (left); 100 µm (right)). Representative images from n = 3 mice. f, Colocalization of Calpain 2 and CDK5 in neuronal cell bodies of the piriform cortex (scale bars, 50 μm (main images); 10 µm (inset)). Representative images from n = 3 mice. g, HSD has no effect on the phosphorylation of the CDK5 substrate DARPP-32 in neocortex; ND/HSD n = 10/10. h, Administration of the CDK5 peptide inhibitor TFP5 has no effect on the increase in serum IL-17 levels induced by HSD (scrambled: ND/HSD n = 5/4, *P = 0.0002 versus ND scrambled; TFP5: ND/HSD n = 7/8, *P < 0.359 versus ND TFP5; two-tailed unpaired t-test). i, l-Arginine does not alter the levels of calpain 1 and 2 in the neocortex or hippocampus. ND/HSD n = 3/5. For gel source data see Supplementary Fig. 1. Data are expressed as mean ± s.e.m.
Extended Data Fig. 5 GSK3β, PIN-1, calpastatin and CDK5 nitrosylation in HSD-fed mice, as well as neurovascular coupling, effect of HJ8.8 on p-tau, serum IL-17, and summary.
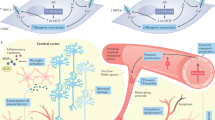
a, HSD has no effect on the expression or activity of GSK3β in the neocortex. ND/HSD n = 10/10. b, HSD does not alter the expression of the prolyl cis/trans isomerase PIN-1, a regulator of tau dephosphorylation. ND/HSD n = 5/5. c, The expression of calpastatin, an endogenous inhibitor of calpain activity, is not reduced by HSD. ND/HSD n = 10/10. d, Nitrosylation of CDK5 is reduced in the neocortex of HSD-fed mice (ND/HSD n = 9/9, diet: *P = 0.0143; ascorbate: *P < 0.0001; two-way ANOVA and Tukey’s test). e, HJ8.8 reduces AT8 in the hippocampus (IgG: ND/HSD n = 13/12; HJ8.8: ND/HSD n = 9/13; *P < 0.0001, Kruskal–Wallis test and Dunn’s test). RZ3 levels are not altered by HJ8.8. f, Administration of HJ8.8 does alter the increase in serum IL-17 levels induced by HSD (IgG: ND/HSD n = 9/9, *P = 0.0192 versus ND IgG; HJ8.8: ND/HSD n = 7/5, *P = 0.0421 versus ND HJ8.8, two-tailed unpaired t-test). g, The increase in somatosensory cortex CBF induced by neural activity evoked by mechanical stimulation of the whiskers is not reduced by HSD in wild-type, tau−/− or HJ8.8-treated mice (wild-type ND/HSD n = 5/7, tau−/− ND/HSD n = 9/8; IgG ND/HSD n = 5/5, HJ8.8 ND/HSD n = 5/5). h, Western blotting showing enrichment of tau in boiled RIPA neocortical samples (heat-stable fraction, HS). Note that β-actin is lost during the boiling process. Representative images from n = 3 experiments. i, Cartoon depicting the mechanisms by which HSD leads to tau phosphorylation and cognitive impairment. HSD elicits a TH17 response in the small intestine, which leads to an increase in circulating IL-17. IL-17, in turn, suppresses endothelial NO production by inducing inhibitory phosphorylation of eNOS at Thr495. The NO deficit results in reduced nitrosylation of calpain in neurons, and increases in calpain activity, p35 to p25 cleavage, activation of CDK5, and tau phosphorylation, which is ultimately responsible for cognitive dysfunction. In support of this chain of events, rescuing the endothelial NO deficit with l-arginine, lack of tau in tau-null mice, treatment with the CDK5 peptide inhibitor TFP5 or treatment with antibodies directed against tau (Tau ab) prevent the cognitive dysfunction. For gel source data see Supplementary Fig. 1. Data are expressed as mean ± s.e.m.
Supplementary information
Supplementary Figures
This file contains full immunoblots images for the figures and extended figures.
Rights and permissions
About this article
Cite this article
Faraco, G., Hochrainer, K., Segarra, S.G. et al. Dietary salt promotes cognitive impairment through tau phosphorylation. Nature 574, 686–690 (2019). https://doi.org/10.1038/s41586-019-1688-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1688-z
This article is cited by
-
Causal relationship between dietary salt intake and dementia risk: Mendelian randomization study
Genes & Nutrition (2024)
-
Dietary salt promotes cognition impairment through GLP-1R/mTOR/p70S6K signaling pathway
Scientific Reports (2024)
-
Post-ischemic ubiquitination at the postsynaptic density reversibly influences the activity of ischemia-relevant kinases
Communications Biology (2024)
-
The underlying mechanisms of DNA methylation in high salt memory in hypertensive vascular disease
Scientific Reports (2024)
-
Meningeal interleukin-17-producing T cells mediate cognitive impairment in a mouse model of salt-sensitive hypertension
Nature Neuroscience (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.