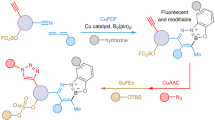
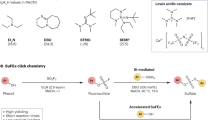
Abstract
Click chemistry is a concept in which modular synthesis is used to rapidly find new molecules with desirable properties1. Copper(i)-catalysed azide–alkyne cycloaddition (CuAAC) triazole annulation and sulfur(vi) fluoride exchange (SuFEx) catalysis are widely regarded as click reactions2,3,4, providing rapid access to their products in yields approaching 100% while being largely orthogonal to other reactions. However, in the case of CuAAC reactions, the availability of azide reagents is limited owing to their potential toxicity and the risk of explosion involved in their preparation. Here we report another reaction to add to the click reaction family: the formation of azides from primary amines, one of the most abundant functional groups5. The reaction uses just one equivalent of a simple diazotizing species, fluorosulfuryl azide6,7,8,9,10,11 (FSO2N3), and enables the preparation of over 1,200 azides on 96-well plates in a safe and practical manner. This reliable transformation is a powerful tool for the CuAAC triazole annulation, the most widely used click reaction at present. This method greatly expands the number of accessible azides and 1,2,3-triazoles and, given the ubiquity of the CuAAC reaction, it should find application in organic synthesis, medicinal chemistry, chemical biology and materials science.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The UPLC chromatograms (UV absorption) of the reaction mixtures for the preparations of the azide compounds in Fig. 2 are available in Supplementary Information 2. The structures of the 1,224 compounds in the azide library, and the UPLC chromatograms of the 1,224 CuAAC reaction mixtures, are also shown in Supplementary Information 2. Information on the source of each amine substrate used is available upon reasonable request from the corresponding authors.
References
Kolb, H. C., Finn, M. G. & Sharpless, K. B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 40, 2004–2021 (2001).
Tornøe, C. W., Christensen, C. & Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 67, 3057–3064 (2002).
Rostovtsev, V. V., Green, L. G., Fokin, V. V. & Sharpless, K. B. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 41, 2596–2599 (2002).
Dong, J., Krasnova, L., Finn, M. G. & Sharpless, K. B. Sulfur(VI) fluoride exchange (SuFEx): another good reaction for click chemistry. Angew. Chem. Int. Ed. 53, 9430–9448 (2014).
Kalliokoski, T. Price-focused analysis of commercially available building blocks for combinatorial library synthesis. ACS Comb. Sci. 17, 600–607 (2015).
Ruff, J. K. Sulfur oxyfluoride derivatives. II. Inorg. Chem. 4, 567–570 (1965).
Shozda, R. J. & Vernon, J. A. Derivatives of azidosulfonic acid. Halides, amides, and salts. J. Org. Chem. 32, 2876–2880 (1967).
Shozda, R. J. Sulfuryl halides and process. US patent 3418088A (1968).
Zeng, X., Gerken, M., Beckers, H. & Willner, H. Anomeric effects in sulfonyl compounds: an experimental and computational study of fluorosulfonyl azide, FSO2N3, and trifluoromethylsulfonyl azide, CF3SO2N3. J. Phys. Chem. A 114, 7624–7630 (2010).
Zeng, X., Beckers, H., Neuhaus, P., Grote, D. & Sander, W. Elusive fluoro sulfinyl nitrite, FS(O)NO, produced by photolysis of matrix-isolated FS(O)2N. Z. Anorg. Allg. Chem. 638, 526–533 (2012).
Zeng, X., Beckers, H. & Willner, H. Thermally persistent fluorosulfonyl nitrene and unexpected formation of the fluorosulfonyl radical. J. Am. Chem. Soc. 135, 2096–2099 (2013).
Cavender, C. J. & Shiner, V. J. Trifluoromethanesulfonyl azide. Its reaction with alkyl amines to form alkyl azides. J. Org. Chem. 37, 3567–3569 (1972).
Alper, P. B., Hung, S.-C. & Wong, C.-H. Metal catalyzed diazo transfer for the synthesis of azides from amines. Tetrahedr. Lett. 37, 6029–6032 (1996).
Nyffeler, P. T., Liang, C.-H., Koeller, K. M. & Wong, C.-H. The chemistry of amine–azide interconversion: catalytic diazotransfer and regioselective azide reduction. J. Am. Chem. Soc. 124, 10773–10778 (2002).
Liu, Q. & Tor, Y. Simple conversion of aromatic amines into azides. Org. Lett. 5, 2571–2572 (2003).
Titz, A., Radic, Z., Schwardt, O. & Ernst, B. A safe and convenient method for the preparation of triflyl azide, and its use in diazo transfer reactions to primary amines. Tetrahedr. Lett. 47, 2383–2385 (2006).
Beckmann, H. S. G. & Wittmann, V. One-pot procedure for diazo transfer and azide–alkyne cycloaddition: triazole linkages from amines. Org. Lett. 9, 1–4 (2007).
Lee, C.-T., Huang, S. & Lipshutz, B. H. Copper-in-charcoal-catalyzed, tandem one-pot diazo transfer-click reactions. Adv. Synth. Catal. 351, 3139–3142 (2009).
Goddard-Borger, E. D. & Stick, R. V. An efficient, inexpensive, and shelf-stable diazotransfer reagent: imidazole-1-sulfonyl azide hydrochloride. Org. Lett. 9, 3797–3800 (2007).
Goddard-Borger, E. D. & Stick, R. V. An efficient, inexpensive, and shelf-stable diazotransfer reagent: imidazole-1-sulfonyl azide hydrochloride. Org. Lett. 13, 2514 (2011).
Guo, T. et al. A new portal to SuFEx click chemistry: a stable fluorosulfuryl imidazolium salt emerging as an “F–SO2+” donor of unprecedented reactivity, selectivity, and scope. Angew. Chem. Int. Ed. 57, 2605–2610 (2018).
Schoffelen, S. et al. Metal-free and pH-controlled introduction of azides in proteins. Chem. Sci. 2, 701–705 (2011).
Glemser, V. O. & Richert, H. Darstellung und eigenschaften von SNF und NSF3. Z. Anorg. Allg. Chem. 307, 313–327 (1961).
Kislukhin, A. A., Hong, V. P., Breitenkamp, K. E. & Finn, M. G. Relative performance of alkynes in copper-catalyzed azide–alkyne cycloaddition. Bioconjug. Chem. 24, 684–689 (2013).
Rees, D. C., Congreve, M., Murray, C. W. & Carr, R. Fragment-based lead discovery. Nat. Rev. Drug Discov. 3, 660–672 (2004).
Murray, C. W. & Rees, D. C. The rise of fragment-based drug discovery. Nat. Chem. 1, 187–192 (2009).
Moffat, J. G., Vincent, F., Lee, J. A., Eder, J. & Prunotto, M. Opportunities and challenges in phenotypic drug discovery: an industry perspective. Nat. Rev. Drug Discov. 16, 531–543 (2017).
Suárez, J. R., Trastoy, B., Pérez-Ojeda, M. E., Barrios, R. M. & Chiara, J. L. Nonafluorobutanesulfonyl azide: a shelf-stable diazo transfer reagent for the synthesis of azides from primary amines. Adv. Synth. Catal. 352, 2515–2520 (2010).
Katritzky, A. R., Khatib, M. E., Shakov, O. B., Khelashvili, L. & Steel, P. J. Benzotriazol-1-yl-sulfonyl azide for diazotransfer and preparation of azidoacylbenzotriazoles. J. Org. Chem. 75, 6532–6539 (2010).
Fischer, N. et al. Sensitivities of some imidazole-1-sulfonyl azide salts. J. Org. Chem. 77, 1760–1764 (2012).
Ye, H. et al. A safe and facile route to imidazole-1-sulfonyl azide as a diazotransfer reagent. Org. Lett. 15, 18–21 (2013).
Stevens, M. Y., Sawant, R. T. & Odell, L. R. Synthesis of sulfonyl azides via diazotransfer using an imidazole-1- sulfonyl azide salt: scope and 15N NMR labeling experiments. J. Org. Chem. 79, 4826–4831 (2014).
Kitamura, M. et al. Direct synthesis of organic azides from primary amines with 2-azido-1,3-dimethylimidazolinium hexafluorophosphate. Eur. J. Org. Chem. 458–462 (2011).
Acknowledgements
We acknowledge the National Natural Science Foundation of China (NSFC 21672240, NSFC 21421002), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB20020300), the Key Research Program of Frontier Sciences (CAS, grant no. QYZDB-SSW-SLH028) and Shanghai Sciences and Technology Committee (18JC1415500, 18401933502) for financial support. We thank J. Yang for help with the thermal and mechanical stability tests; K. Ding for support; and P. Gao, T. Wang, Y. Liang, X. Zhan and J. Chen for assistance with the management of the amine compounds.
Author information
Authors and Affiliations
Contributions
G.M. developed the procedure for the preparation of fluorosulfuryl azide. T.G., G.M., Y.S. and T.M. prepared the 1,224-amine library. G.M. and T.G. developed the procedure for the diazotransfer reaction. T.G., T.M., J.Z., Y.S. and G.M. synthesized the isolated azide compounds. T.G. prepared the 1,224-azide library. T.M. carried out the CuAAC reaction with the 1,224-azide library. G.M. and T.G. analysed the UPLC–MS chromatograms of the CuAAC reactions. J.D. and K.B.S. supervised the project and conceived the style of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Shanghai Institute of Organic Chemistry, CAS has filed patent applications for this technology (Chinese patent application numbers PCT/CN2018/116922, CN201810609851.4 and CN201810609157.2).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peer review information Nature thanks Joseph Topczewski and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Supplementary information
Supplementary Information 1
This file contains the detailed procedures for the preparation of the compounds mentioned in the paper. Characterization data for the isolated compounds and the chemical structures for the 1,224-azide compound library are also provided.
Supplementary Information 2
This file contains the illustrated UPLC chromatograms for the reaction mixtures of all the copper(i)-catalyzed azide-alkyne cycloaddition (CuAAC) reactions carried out with the azide compound library.
Supplementary Information 3
This file provides the original study report for the acute oral toxicity of 1-(fluorosulfuryl)-2,3-dimethyl-1H-imidazol-3-ium trifluoromethanesulfonate (compound 1 in this paper) in SD rats.
Rights and permissions
About this article
Cite this article
Meng, G., Guo, T., Ma, T. et al. Modular click chemistry libraries for functional screens using a diazotizing reagent. Nature 574, 86–89 (2019). https://doi.org/10.1038/s41586-019-1589-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1589-1
This article is cited by
-
A general strategy to develop fluorogenic polymethine dyes for bioimaging
Nature Chemistry (2024)
-
Electrostatic catalysis of a click reaction in a microfluidic cell
Nature Communications (2024)
-
Radiochemistry for positron emission tomography
Nature Communications (2023)
-
Sulfur fluoride exchange
Nature Reviews Methods Primers (2023)
-
Combined radical and ionic approach for the enantioselective synthesis of β-functionalized amines from alcohols
Nature Synthesis (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.