Abstract
The causative agent of plague, Yersinia pestis, uses a type III secretion system to selectively destroy immune cells in humans, thus enabling Y. pestis to reproduce in the bloodstream and be transmitted to new hosts through fleabites. The host factors that are responsible for the selective destruction of immune cells by plague bacteria are unknown. Here we show that LcrV, the needle cap protein of the Y. pestis type III secretion system, binds to the N-formylpeptide receptor (FPR1) on human immune cells to promote the translocation of bacterial effectors. Plague infection in mice is characterized by high mortality; however, Fpr1-deficient mice have increased survival and antibody responses that are protective against plague. We identified FPR1R190W as a candidate resistance allele in humans that protects neutrophils from destruction by the Y. pestis type III secretion system. Thus, FPR1 is a plague receptor on immune cells in both humans and mice, and its absence or mutation provides protection against Y. pestis. Furthermore, plague selection of FPR1 alleles appears to have shaped human immune responses towards other infectious diseases and malignant neoplasms.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Andrades Valtueña, A. et al. The Stone Age plague and its persistence in Eurasia. Curr. Biol. 27, 3683–3691 (2017).
Stenseth, N. C. et al. Plague: past, present, and future. PLoS Med. 5, e3 (2008).
Benedictow, O. J. The Black Death 1346–1353: The Complete History (Boydell, 2004).
Samson, M. et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382, 722–725 (1996).
Liu, R. et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86, 367–377 (1996).
Stephens, J. C. et al. Dating the origin of the CCR5-Δ32 AIDS-resistance allele by the coalescence of haplotypes. Am. J. Hum. Genet. 62, 1507–1515 (1998).
Mecsas, J. et al. CCR5 mutation and plague protection. Nature 427, 606 (2004).
Elvin, S. J. et al. Ambiguous role of CCR5 in Y. pestis infection. Nature 430, 418 (2004).
Galán, J. E., Lara-Tejero, M., Marlovits, T. C. & Wagner, S. Bacterial type III secretion systems: specialized nanomachines for protein delivery into target cells. Annu. Rev. Microbiol. 68, 415–438 (2014).
Achtman, M. et al. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc. Natl Acad. Sci. USA 96, 14043–14048 (1999).
Cornelis, G. R. The Yersinia deadly kiss. J. Bacteriol. 180, 5495–5504 (1998).
Marketon, M. M., DePaolo, R. W., DeBord, K. L., Jabri, B. & Schneewind, O. Plague bacteria target immune cells during infection. Science 309, 1739–1741 (2005).
Inglesby, T. V. et al. Plague as a biological weapon: medical and public health management. J. Am. Med. Assoc. 283, 2281–2290 (2000).
Quenee, L. E. & Schneewind, O. Plague vaccines and the molecular basis of immunity against Yersinia pestis. Hum. Vaccin. 5, 817–823 (2009).
Mitchell, A. et al. Glutathionylation of Yersinia pestis LcrV and its efects on plague pathogenesis. mBio 8, e00646-17 (2017).
Collier, R. J. Understanding the mode of action of diphtheria toxin: a perspective on progress during the 20th century. Toxicon 39, 1793–1803 (2001).
Boulay, F., Tardif, M., Brouchon, L. & Vignais, P. The human N-formylpeptide receptor. Characterization of two cDNA isolates and evidence for a new subfamily of G-protein-coupled receptors. Biochemistry 29, 11123–11133 (1990).
Miettinen, H. M. et al. The ligand binding site of the formyl peptide receptor maps in the transmembrane region. J. Immunol. 159, 4045–4054 (1997).
Le, Y., Murphy, P. M. & Wang, J. M. Formyl-peptide receptors revisited. Trends Immunol. 23, 541–548 (2002).
Schaffrath, R. & Stark, M. J. Decoding the biosynthesis and function of diphthamide, an enigmatic modification of translation elongation factor 2 (EF2). Microb. Cell 1, 203–205 (2014).
Sheahan, K.-L. & Isberg, R. R. Identification of mammalian proteins that collaborate with type III secretion system function: involvement of a chemokine receptor in supporting translocon activity. mBio 6, e02023-14 (2015).
Bardoel, B. W. et al. Identification of an immunomodulating metalloprotease of Pseudomonas aeruginosa (IMPa). Cell. Microbiol. 14, 902–913 (2012).
Mueller, C. A. et al. The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science 310, 674–676 (2005).
Vacchelli, E. et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science 350, 972–978 (2015).
Perretti, M. & D’Acquisto, F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 9, 62–70 (2009).
Postma, B. et al. Chemotaxis inhibitory protein of Staphylococcus aureus binds specifically to the C5a and formylated peptide receptor. J. Immunol. 172, 6994–7001 (2004).
Wenzel-Seifert, K., Grünbaum, L. & Seifert, R. Differential inhibition of human neutrophil activation by cyclosporins A, D, and H. Cyclosporin H is a potent and effective inhibitor of formyl peptide-induced superoxide formation. J. Immunol. 147, 1940–1946 (1991).
Ligtenberg, K. G., Miller, N. C., Mitchell, A., Plano, G. V. & Schneewind, O. LcrV mutants that abolish Yersinia type III injectisome function. J. Bacteriol. 195, 777–787 (2013).
Welkos, S., Friedlander, A., McDowell, D., Weeks, J. & Tobery, S. V antigen of Yersinia pestis inhibits neutrophil chemotaxis. Microb. Pathog. 24, 185–196 (1998).
Bartra, S. S., Jackson, M. W., Ross, J. A. & Plano, G. V. Calcium-regulated type III secretion of Yop proteins by an Escherichia coli hha mutant carrying a Yersinia pestis pCD1 virulence plasmid. Infect. Immun. 74, 1381–1386 (2006).
Gage, K. L. & Kosoy, M. Y. Natural history of plague: perspectives from more than a century of research. Annu. Rev. Entomol. 50, 505–528 (2005).
Hartt, J. K., Barish, G., Murphy, P. M. & Gao, J. L. N-formylpeptides induce two distinct concentration optima for mouse neutrophil chemotaxis by differential interaction with two N-formylpeptide receptor (FPR) subtypes. Molecular characterization of FPR2, a second mouse neutrophil FPR. J. Exp. Med. 190, 741–748 (1999).
Stempel, H. et al. Strain-specific loss of formyl peptide receptor 3 in the murine vomeronasal and immune systems. J. Biol. Chem. 291, 9762–9775 (2016).
Liu, M. et al. Formylpeptide receptors are critical for rapid neutrophil mobilization in host defense against Listeria monocytogenes. Sci. Rep. 2, 786 (2012).
Pechous, R. D., Sivaraman, V., Price, P. A., Stasulli, N. M. & Goldman, W. E. Early host cell targets of Yersinia pestis during primary pneumonic plague. PLoS Pathog. 9, e1003679 (2013).
Shannon, J. G. et al. Yersinia pestis subverts the dermal neutrophil response in a mouse model of bubonic plague. mBio 4, e00170-13 (2013).
Quenee, L. E., Cornelius, C. A., Ciletti, N. A., Elli, D. & Schneewind, O. Yersinia pestis caf1 variants and the limits of plague vaccine protection. Infect. Immun. 76, 2025–2036 (2008).
Sahagun-Ruiz, A. et al. Contrasting evolution of the human leukocyte N-formylpeptide receptor subtypes FPR and FPRL1R. Genes Immun. 2, 335–342 (2001).
Håkansson, S., Bergman, T., Vanooteghem, J.-C., Cornelis, G. & Wolf-Watz, H. YopB and YopD constitute a novel class of Yersinia Yop proteins. Infect. Immun. 61, 71–80 (1993).
Torruellas, J., Jackson, M. W., Pennock, J. W. & Plano, G. V. The Yersinia pestis type III secretion needle plays a role in the regulation of Yop secretion. Mol. Microbiol. 57, 1719–1733 (2005).
Cornelis, G. R. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158, 401–408 (2002).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010).
Dorward, D. A. et al. The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation. Am. J. Pathol. 185, 1172–1184 (2015).
Muto, Y., Guindon, S., Umemura, T., Kőhidai, L. & Ueda, H. Adaptive evolution of formyl peptide receptors in mammals. J. Mol. Evol. 80, 130–141 (2015).
Ye, R. D. et al. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 61, 119–161 (2009).
Quignon, P., Rimbault, M., Robin, S. & Galibert, F. Genetics of canine olfaction and receptor diversity. Mamm. Genome 23, 132–143 (2012).
Perry, R. D. & Fetherston, J. D. Yersinia pestis—etiologic agent of plague. Clin. Microbiol. Rev. 10, 35–66 (1997).
Baeten, L. A. et al. Immunological and clinical response of coyotes (Canis latrans) to experimental inoculation with Yersinia pestis. J. Wildl. Dis. 49, 932–939 (2013).
Liang, X. Y. et al. FPR1 interacts with CFH, HTRA1 and smoking in exudative age-related macular degeneration and polypoidal choroidal vasculopathy. Eye 28, 1502–1510 (2014).
Otani, T. et al. Polymorphisms of the formylpeptide receptor gene (FPR1) and susceptibility to stomach cancer in 1531 consecutive autopsy cases. Biochem. Biophys. Res. Commun. 405, 356–361 (2011).
Girard, G. Plague. Annu. Rev. Microbiol. 9, 253–276 (1955).
Meyer, K. F., Smith, G., Foster, L., Brookman, M. & Sung, M. H. Live, attenuated Yersinia pestis vaccine: virulent in non-human primates, harmless to guinea pigs. J. Infect. Dis. 129, S85–S120 (1974).
Brubaker, R. R. Mutation rate to nonpigmentation in Pasteurella pestis. J. Bacteriol. 98, 1404–1406 (1969).
DeBord, K. L. et al. Immunogenicity and protective immunity against bubonic plague and pneumonic plague by immunization of mice with the recombinant V10 antigen, a variant of LcrV. Infect. Immun. 74, 4910–4914 (2006).
Higuchi, K. An improved chemically defined culture medium for strain L mouse cells based on growth responses to graded levels of nutrients including iron and zinc ions. J. Cell. Physiol. 75, 65–72 (1970).
Doll, J. M. et al. Cat-transmitted fatal pneumonic plague in a person who traveled from Colorado to Arizona. Am. J. Trop. Med. Hyg. 51, 109–114 (1994).
Hanahan, D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166, 557–580 (1983).
Forsberg, A., Bölin, I., Norlander, L. & Wolf-Watz, H. Molecular cloning and expression of calcium-regulated, plasmid-coded proteins of Y. pseudotuberculosis. Microb. Pathog. 2, 123–137 (1987).
Ton-That, H. & Schneewind, O. Assembly of pili on the surface of Corynebacterium diphtheriae. Mol. Microbiol. 50, 1429–1438 (2003).
Anderson, D. M. & Schneewind, O. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science 278, 1140–1143 (1997).
Sundström, C. & Nilsson, K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int. J. Cancer 17, 565–577 (1976).
Gallagher, R. et al. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood 54, 713–733 (1979).
Shalem, O. et al. Genome-scale CRISPR–Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Edgar, R., Domrachev, M. & Lash, A. E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002).
Quenee, L. E., Ciletti, N. A., Elli, D., Hermanas, T. M. & Schneewind, O. Prevention of pneumonic plague in mice, rats, guinea pigs and non-human primates with clinical grade rV10, rV10-2 or F1-V vaccines. Vaccine 29, 6572–6583 (2011).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011).
Acknowledgements
We thank J. A. van Strijp for providing CHIPS, G. V. Plano and D. Anderson for sharing and providing strains Y. pestis KIM 8 and KIM 8(Δ1234), P. M. Murphy for providing breeding pairs of FPR1 and FPR2 mice, C. Tam and D. Elli for experimental assistance, J. Andrade and Y. Li for assistance with bioinformatics analysis of sequence data, C. Ober, T. Golovkina and members of our laboratory for discussion. O.S. is grateful to P. Model for his lifelong support and friendship. This project has been supported by funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under awards AI107792, AI057153 and AI042797.
Author information
Authors and Affiliations
Contributions
P.O.-O., T.M.C., H.K.K., D.M. and O.S. developed methods and conceptualized ideas; P.O.-O., T.M.C., H.K.K., D.M. and O.S. designed experiments; P.O.-O., T.M.C., H.K.K. and O.S. performed experiments; P.O.-O., T.M.C., H.K.K., D.M. and O.S. analysed data; P.O.-O., T.M.C., D.M. and O.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peer review information Nature thanks Igor E. Brodsky, Ralph Isberg, Adriano G. Rossi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Fig. 1 Generation of FPR1−/− U937 cells using CRISPR–Cas9 and genetic complementation.
a, Sequencing results for alleles cloned from CRISPR–Cas9-derived FPR1−/− U937 macrophages. b, Immunoblot analysis for the production of FPR1, FPR2 and FPR3 in U937 macrophages, CRISPR–Cas9-derived FPR1−/− cells and FPR1−/− cells transfected with FPR1. Numbers to the left of the blots indicate migration of molecular weight markers. One of three repeats is shown (a, b).
Extended Data Fig. 2 Contribution of CCR5 to Yersinia spp. intoxication and injection by the T3SS.
a, Sequencing results for the alleles of two CCR5−/− U937 isolates obtained using CRISPR–Cas9 mutagenesis. b, Cell survival following incubation with Y. pestis strains KIM D27 and POO1 was measured using the Trypan blue exclusion assay. Data are mean ± s.e.m. (n = 4 biological replicates, shown as circles). c, d, T3SS injection into U937, FPR1−/− and CCR5−/− cells by Y. pestis KIM D27 (c) and Y. pseudotuberculosis YPIII carrying pMM83 (YopM–Bla) (d). Data are mean ± s.e.m. (n = 3 biological replicates, shown as circles). One of three repeats is shown (b–d). Statistical analysis was performed using one-way ANOVA with Bonferroni correction. ***P < 0.001; **P < 0.01.
Extended Data Fig. 3 Antibodies and ligands of FPR1 inhibit Y. pestis type III secretion in human neutrophils.
a, b, Human neutrophils were stained with the β-lactamase substrate CCF2-AM, infected with wild-type (WT) Y. pestis KIM D27(pMM83) or the KLD29 variant (ΔlcrV, pMM83), which is defective for the T3SS, and analysed for blue fluorescence (a; YopM–Bla translocation into neutrophils and CCF2-AM cleavage) or green fluorescence (b) to derive the percentage of stained cells injected with the T3SS effector. c, Inhibition of the Y. pestis T3SS in human neutrophils by monoclonal and polyclonal antibodies against FPR1, bacterial LcrV, fMLF, annexin A1 peptide, staphylococcal CHIPS and cyclosporin H. d, Differential interference contrast (DIC) and fluorescence microscopy of mock or fMLF-treated differentiated U937 cells infected with green-fluorescent Y. pestis KIM D27(eGFP) and stained with F1-specific antibody (red) to reveal extracellular bacteria in merged images of mock, but not in merged images of fMLF-treated cells. Orange and green arrows indicate extracellular and intracellular bacteria, respectively. e, Antibodies and ligands of FPR1 inhibit the Y. pestis T3SS of YopM–Bla in U937 macrophages. One of three repeats is shown (a–e). Data are mean + s.e.m. (n = 3 biological replicates, shown as circles) (c, e).One-way ANOVA with Bonferroni correction was used to identify significant differences (c, e). ***P < 0.001; **P < 0.01.
Extended Data Fig. 4 Screening 45 monoclonal antibodies for inhibition of the Y. pestis T3SS in human neutrophils.
Human neutrophils were stained with the β-lactamase substrate CCF2-AM (green fluorescence), and infected with wild-type Y. pestis KIM D27(pMM83). Translocation of YopM–Bla into neutrophils results in CCF2-AM cleavage (blue fluorescence) allowing for quantification of the percentage of stained cells injected with the T3SS effector in the absence (mock) or presence of specific monoclonal antibodies. Data are mean + s.e.m. (n = 3 biological replicates, shown as circles). A representative experiment of three independent experiments is shown.
Extended Data Fig. 5 FPR1−/− cells migrate towards chemoattractants other than formylated peptides and differentiated HL-60 cells migrate towards Y. pestis.
a, Numbers of migrating immune cells were quantified in a Transwell assay primed with mock, 10 nM fMLF, 10 nM LTB4 or 100 ng ml−1 KC (CXCL1) for U937, FPR1−/− and FPR1−/−(FPR1) cells. b, Numbers of migrating HL-60 cells were quantified in a Transwell assay primed with mock, Y. pestis KIM D27(WT) or KLD29(ΔlcrV) (107 CFU ml−1). Chemotaxis towards fMLF is shown as a control. c, Increasing concentrations of LcrVS228 (10−1–103 ng ml−1) were added to the Transwell assay and number of migrating HL-60 cells recorded. Data are mean + s.e.m. (n = 3 biological replicates, shown as circles); one-way ANOVA with Bonferroni correction was used to identify significant differences. ***P < 0.001; **P < 0.01; *P < 0.05; ns, not significant. A representative of three independent experiments is shown.
Extended Data Fig. 6 Adhesion of Y. pestis to human macrophages does not require effector Yops and loss of FPR1 in human macrophages can be complemented with FPR1STREP.
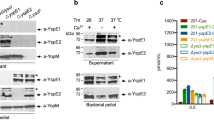
a, Y. pestis KIM 8 and its variant KIM 8(Δ1234) (Yop-less) were grown at 37 °C in thoroughly modified Higuchi’s medium to induce the T3SS. Cultures were centrifuged to separate the supernatant from the bacterial pellet, and extracts were analysed by immunoblotting with antibodies specific to YopB (αYopB), YopD (αYopD), YopE (αYopE), LcrV (αLcrV), YscF (αYscF), YopH (αYopH), YopM (αYopM) and YopJ (αYopJ). One of three repeats is shown. b, Wild-type Y. pestis KIM 8 or its Yop-less variant, KIM 8(Δ1234) were added to U937 and FPR1−/− cells (MOI of 10) and adherence was quantified as the percentage inoculum. Data are mean + s.e.m. (n = 3 biological replicates, shown as circles). One-way ANOVA with Bonferroni correction was used to identify significant differences. ∗∗P < 0.01. A representative experiment of three independent experiments is shown. c, d, Genetic complementation in FPR1−/− cells using FPR1STREP. c, Cell lysis induced by Y. pestis POO1 (yopE-dtx) but not POO2 (ΔlcrV, yopE-dtx) is restored in FPR1−/− cells transfected with a plasmid expressing C-terminal Strep-tag II FPR1 (FPR1STREP). d, Y. pestis KIM D27(pMM83) mediated YopM–Bla translocation into U937, FPR1−/− and FPR1−/−(FPR1STREP) cells. One of three repeats is shown. Data are mean + s.e.m. (n = 3 biological replicates, shown as circles) (c, d). Significant differences were measured with one-way ANOVA with Bonferroni post hoc analysis. ***P < 0.001.
Extended Data Fig. 7 T3SS injection of Fpr1−/− neutrophils in vitro and in vivo.
a, b, Mouse plasma does not affect Fpr1−/− neutrophil resistance to T3SS injection. Y. pestis KIM D27(pMM83) translocation of YopM–Bla in mouse neutrophils, incubated in mouse plasma with (a) or without (b) prior heat inactivation (HI). One of three repeats is shown. Data are mean + s.e.m. (n = 3 biological replicates, shown as circles). One-way ANOVA with Bonferroni correction was used to identify significant differences. ***P < 0.001; **P < 0.01. c–e, The Fpr1−/− mutation does not abolish the influx of neutrophils into Y.-pestis-infected tissues. Age- and sex-matched C57BL/6J and Fpr1−/− mice (4 groups, n = 5 per group, 6–8 weeks old, 5 males and 5 females, 2 experimental replicates) were anaesthetized and infected by injection of 1,000 CFU Y. pestis CO92 into the inguinal region. Then, 4 h after the challenge, euthanized mice were necropsied, the dermis surrounding the injection site was removed and fixed in formalin for histopathological analysis. Consecutive thin-sectioned slides were stained with haematoxylin and eosin (H&E) or stained by immunohistochemistry with the neutrophil marker anti-Ly6G (α-Ly6G). c, Slides were analysed by a blinded investigator and assigned one of four pathology scores: 0, no neutrophil influx; 1, local infiltration; 2, moderate local infiltration; 3, widespread infiltration. d, e, Analysis of neutrophil influx was performed in male (n = 8) and in female (n = 10) mice (d) or in all mice (e; male and female, n = 18; 2 mice were excluded from the analysis owing to unclear histology). One-way ANOVA with Bonferroni correction was used to analyse differences. ns, not significant (d, e).
Extended Data Fig. 8 Contribution of mouse FRP1 to plague disease.
a, Serum derived from naive or Y.-pestis-infected Fpr1−/− mice (experiment shown in Fig. 4e) was analysed for IgG specific to capsular fraction 1 antigen (αF1) using ELISA. One of three repeats is shown. Data are mean + s.e.m. (n = 3 biological replicates, shown as circles). b, Representative spleen sections stained with haematoxylin and eosin from naive or Y.-pestis-infected mice shown in Fig. 4e. c, Measurements of white pulp areas in spleens. Each dot represents the average white pulp surface area in each mouse (n = 19–101 per mouse, data quantified using ImageJ). Horizontal bars denote the mean, circles indicate individual values. Significant differences were determined using one-way ANOVA and Bonferroni post hoc analysis (a) and Mann–Whitney and Kruskal–Wallis tests (c). **P < 0.01; *P < 0.05; ns, not significant
Extended Data Fig. 9 Genetic and functional analyses of FPR1 and CCR5 genes from five human donors.
a, Serum derived from the blood of naive or Y.-pestis-infected non-human primates (NHP, cynomolgus macaque) or human blood donors described in Fig. 5a (donors 1–5) was analysed for IgG specific to F1 antigen using ELISA. b, List of amino acids changes deduced following cloning and sequencing of FPR1 and CCR5 alleles from human blood neutrophils (donors 1–5). c, Quantification of Y. pestis KIM D27(pMM83) translocation of YopM–Bla into U937 or FPR1−/− macrophages transfected with plasmids FPR1 and FPR1(R190W). d, Immunoblot analysis for the production of FPR1, FPR2, FPR3 and actin in U937 macrophages, and derived FPR1−/− cells untransfected or transfected with FPR1 and FPR1(R190W). One of three repeats is shown (a, c). Data are mean + s.e.m. (n = 3 biological replicates, shown as circles) (a, c). One-way ANOVA and Bonferroni post hoc analyses (c) were used to identify significant differences. ***P < 0.001.
Extended Data Fig. 10 Model summarizing Y. pestis interactions with the plague receptor on human immune cells.
a, Y. pestis releases N-formylpeptides via its T3SS to attract human neutrophils by activating FPR1 signalling and chemotaxis. b, The Y. pestis T3SS docks on the plague receptor (FPR1) via the LcrV needle cap protein. c, Docking promotes assembly of the membrane translocon (including LcrV, YopD and YopB), which provides a conduit for low-calcium signalling to the bacterial T3SS. d, Low-calcium signalling activates T3SS transport of Yop effectors into the cytoplasm, thereby killing host immune cells. e, LcrV shares homology with the annexin A1 peptide. Alignment performed using Clustal Omega66.
Supplementary information
Supplementary Table
This file contains Supplementary Table 1
Supplementary Figures
This file contains source data for blots.
Supplementary Data
This file contains Supplementary Data Set 1: CRISPR-Cas9 screen 1 for Y. pestis POO1-resistant U937 cells.
Supplementary Data
This file contains Supplementary Data Set 2: CRISPR-Cas9 screen 2 for Y. pestis POO1-resistant U937 cells.
Supplementary Data
This file contains Supplementary Data Set 3: CRISPR-Cas9 screen 3 for Y. pestis POO1-resistant U937 cells.
Rights and permissions
About this article
Cite this article
Osei-Owusu, P., Charlton, T.M., Kim, H.K. et al. FPR1 is the plague receptor on host immune cells. Nature 574, 57–62 (2019). https://doi.org/10.1038/s41586-019-1570-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1570-z
This article is cited by
-
Annexins—a family of proteins with distinctive tastes for cell signaling and membrane dynamics
Nature Communications (2024)
-
SNCA correlates with immune infiltration and serves as a prognostic biomarker in lung adenocarcinoma
BMC Cancer (2022)
-
Minimal gene set discovery in single-cell mRNA-seq datasets with ActiveSVM
Nature Computational Science (2022)
-
Immunogenic cell stress and death
Nature Immunology (2022)
-
Molecular recognition of formylpeptides and diverse agonists by the formylpeptide receptors FPR1 and FPR2
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.