Abstract
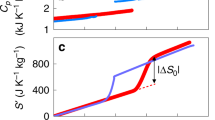
Refrigeration is of vital importance for modern society—for example, for food storage and air conditioning—and 25 to 30 per cent of the world’s electricity is consumed for refrigeration1. Current refrigeration technology mostly involves the conventional vapour compression cycle, but the materials used in this technology are of growing environmental concern because of their large global warming potential2. As a promising alternative, refrigeration technologies based on solid-state caloric effects have been attracting attention in recent decades3,4,5. However, their application is restricted by the limited performance of current caloric materials, owing to small isothermal entropy changes and large driving magnetic fields. Here we report colossal barocaloric effects (CBCEs) (barocaloric effects are cooling effects of pressure-induced phase transitions) in a class of disordered solids called plastic crystals. The obtained entropy changes in a representative plastic crystal, neopentylglycol, are about 389 joules per kilogram per kelvin near room temperature. Pressure-dependent neutron scattering measurements reveal that CBCEs in plastic crystals can be attributed to the combination of extensive molecular orientational disorder, giant compressibility and highly anharmonic lattice dynamics of these materials. Our study establishes the microscopic mechanism of CBCEs in plastic crystals and paves the way to next-generation solid-state refrigeration technologies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
United Nations Environment Programme. The Importance of Energy Efficiency in the Refrigeration, Air-Conditioning and Heat Pump Sectors. Briefing Note A http://conf.montreal-protocol.org/meeting/workshops/energy-efficiency/presession/breifingnotes/briefingnote-a_importance-of-energy-efficiency-in-the-refrigeration-air-conditioning-and-heat-pump-sectors.pdf (United Nations Environment Programme, May 2018).
Molenbroek, E. et al. Savings and Benefits of Global Regulations for Energy Efficient Products. Final Report https://ec.europa.eu/energy/sites/ener/files/documents/Cost%20of%20Non-World%20-%20Final%20Report.pdf (European Commission, 2015).
Shen, B. G., Sun, J. R., Hu, F. X., Zhang, H. W. & Cheng, Z. H. Recent progress in exploring magnetocaloric materials. Adv. Mater. 21, 4545–4564 (2009).
Scott, J. F. Electrocaloric materials. Annu. Rev. Mater. Res. 41, 229–240 (2011).
Mañosa, L. et al. Materials with giant mechanocaloric effects: cooling by strength. Adv. Mater. 29, 1603607 (2017).
Li, B. et al. Magnetostructural coupling and magnetocaloric effect in Ni–Mn–In. Appl. Phys. Lett. 95, 172506 (2009).
Li, B. et al. Intrinsic electrocaloric effects in ferroelectric poly(vinylidene fluoride-trifluoroethylene) copolymers: roles of order of phase transition and stresses. Appl. Phys. Lett. 96, 102903 (2010).
Cazorla, C. & Errandonea, D. Giant mechanocaloric effects in fluorite-structured superionic materials. Nano Lett. 16, 3124–3129 (2016).
Aznar, A. et al. Giant barocaloric effects over a wide temperature range in superionic conductor AgI. Nat. Commun. 8, 1851 (2017).
Bom, N. M. et al. Giant barocaloric effects in natural rubber: a relevant step toward solid-state cooling. ACS Macro Lett. 7, 31–36 (2018).
Parsonage, N. G. & Staveley, L. A. K. Disorder in Crystals (Clarendon Press, Oxford, 1979).
Chandra, D. et al. Phase transitions in “plastic crystals”. J. Less Common Met. 168, 159–167 (1991).
Murrill, E. et al. Solid–solid phase transitions determined by differential scanning calorimetry: part I. Tetrahedral substances. Thermochim. Acta 1, 239–246 (1970).
Tamarit, J. L. et al. Dielectric studies on orientationally disordered phases of neopentylglycol ((CH3)2C(CH2OH)2) and tris(hydroxymethyl aminomethane) ((NH2)C(CH2OH)3). J. Phys. Condens. Matter 9, 5469–5478 (1997).
Desai, P. D. Thermodynamic properties of iron and silicon. J. Phys. Chem. Ref. Data 15, 967–983 (1986).
Desai, P. D. Thermodynamic properties of manganese and molybdenum. J. Phys. Chem. Ref. Data 16, 91–108 (1987).
Desai, P. D. Thermodynamic properties of titanium. Int. J. Thermophys. 8, 781–794 (1987).
Yamaguchi, K. et al. Measurements of high temperature heat content of the II–VI and IV–VI (II: Zn, Cd IV: Sn, Pb VI: Se, Te) compounds. Mater. Trans. 35, 118–124 (1994).
Timmermans, J. Un nouvel état mésomorphe les cristaux organiques plastiques. J. Chim. Phys. 35, 331–344 (1938).
Font, J. et al. Plastic crystals: dilatometric and thermobarometric complementary studies. Mater. Res. Bull. 30, 839–844 (1995).
Webelements http://www.webelements.com/.
Bansal, D. et al. Phonon anharmonicity and negative thermal expansion in SnSe. Phys. Rev. B 94, 054307 (2016).
Hanneman, R. E. et al. Relationship between compressibility and thermal expansion coefficients in cubic metals and alloys. J. Appl. Phys. 36, 1794–1796 (1965); erratum 38, 1988 (1967).
Mañosa, L. et al. Giant solid-state barocaloric effect in the Ni–Mn–In magnetic shape-memory alloy. Nat. Mater. 9, 478–481 (2010).
Lloveras, P. et al. Giant barocaloric effects at low pressure in ferrielectric ammonium sulphate. Nat. Commun. 6, 8801 (2015).
Bermúdez-García, J. M. et al. Giant barocaloric effect in the ferroic organic-inorganic hybrid [TPrA][Mn(dca)3] perovskite under easily accessible pressures. Nat. Commun. 8, 15715 (2017).
Trung, N. T., Zhang, L., Caron, L., Buschow, K. H. J. & Brück, E. Giant magnetocaloric effects by tailoring the phase transitions. Appl. Phys. Lett. 96, 172504 (2010).
Lu, S. G. et al. Comparison of directly and indirectly measured electrocaloric effect in relaxor ferroelectric polymers. Appl. Phys. Lett. 97, 202901 (2010).
Cui, J. et al. Demonstration of high efficiency elastocaloric cooling with large ΔT using NiTi wires. Appl. Phys. Lett. 101, 073904 (2012).
Chandra, D. et al. Low- and high-temperature structures of neopentylglycol plastic crystal. Powder Diffr. 8, 109–117 (1993).
Grüneisen, E. Theorie des festen zustandes einatomiger elemente. Ann. Phys. 344, 257–306 (1912).
Wang, X., Guo, Q., Zhong, Y., Wei, X. & Liu, L. Heat transfer enhancement of neopentyl glycol using compressed expanded natural graphite for thermal energy storage. Renew. Energy 51, 241–246 (2013).
Stern-Taulats, E. et al. Caloric effects in ferroic materials. MRS Bull. 43, 295–299 (2018).
Moya, X. et al. Caloric materials near ferroic phase transitions. Nat. Mater. 13, 439–450 (2014).
Chandra, D. et al. Heat capacities of “plastic crystal” solid state thermal energy storage materials. Z. Phys. Chem. 216, 1433 (2002).
Kawaguchi, S. et al. High-throughput powder diffraction measurement system consisting of multiple MYTHEN detectors at beamline BL02B2 of SPring-8. Rev. Sci. Instrum. 88, 085111 (2017).
Petříček, V., Dušek, M. & Palatinus, L. Crystallographic computing system JANA2006: general features. Z. Kristallogr. 229, 345–352 (2014).
Isshiki, M., Ohishi, Y., Goto, S., Takeshita, K. & Ishikawa, T. High-energy X-ray diffraction beamline: BL04B2 at SPring-8. Nucl. Instrum. Meth. Phys. Res. B 467–468, 663–666 (2001).
Wu, C.-M. et al. SIKA–the multiplexing cold-neutron triple-axis spectrometer at ANSTO. J. Inst. 11, P10009 (2016).
Nakajima, K. et al. AMATERAS: a cold-neutron disk chopper spectrometer. J. Phys. Soc. Jpn 80, SB028 (2011).
Nakamura, M. et al. First demonstration of novel method for inelastic neutron scattering measurement utilizing multiple incident energies. J. Phys. Soc. Jpn 78, 093002 (2009).
Inamura, Y. et al. Development status of software “Utsusemi” for chopper spectrometers at MLF, J-PARC. J. Phys. Soc. Jpn 82, SA031 (2013).
Azuah, R. T. et al. DAVE: a comprehensive software suite for the reduction, visualization, and analysis of low energy neutron spectroscopic data. J. Res. Natl Inst. Stand. Technol. 114, 341–358 (2009).
Aso, N., Fujiwara, T., Uwatoko, Y., Miyano, H. & Yoshizawa, H. Development of a hybrid CuBe/NiCrAl clamp-type high pressure cell for neutron diffraction. J. Phys. Soc. Jpn 76, 228–229 (2007).
Hattori, T. et al. Design and performance of high-pressure PLANET beamline at pulsed neutron source at J-PARC. Nucl. Instrum. Methods Phys. Res. A 780, 55–67 (2015).
Dewaele, A. et al. High-pressure-high-temperature equation of state of KCl and KBr. Phys. Rev. B 85, 214105 (2012).
Yu, D. et al. Performance test on PELICAN – a multi-purpose time of flight cold neutron spectrometer. EPJ Web Conf. 83, 03019 (2015).
Yu, D. et al. Pelican – a time of flight cold neutron polarization analysis spectrometer at OPAL. J. Phys. Soc. Jpn 82, SA027 (2013).
Richard, D. et al. Analysis and visualisation of neutron-scattering data. J. Neutr. Res. 4, 33–39 (1996).
Bée, M. Quasielastic Neutron Scattering Principles and Applications in Solid State Chemistry, Biology and Materials Science (IOP, Bristol, 1988).
Silvi, L., Röhm, E., Fichtner, M., Petry, W. & Lohstroh, W. Hydrogen dynamics in β-Mg(BH4)2 on the picosecond timescale. Phys. Chem. Chem. Phys. 18, 14323–14332 (2016).
Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 117, 1–19 (1995).
Jorgensen, W. L., Maxwell, D. S. & Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118, 11225–11236 (1996).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: An N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Nosé, S. A. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 81, 511–519 (1984).
Hoover, W. G. Canonical dynamics: equilibrium phase-space distributions. Phys. Rev. A 31, 1695–1697 (1985).
Kresse, G. et al. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. et al. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Perdew, J. P. et al. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996); erratum 78, 1396 (1997).
Kresse, G. et al. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Elsässer, C. et al. Density-functional energies and forces with Gaussian-broadened fractional occupations. Phys. Rev. B 49, 13975–13978 (1994).
Grimme, S. et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Svitelskiy, O. et al. Elastic properties of Gd5Si2Ge2 studied with an ultrasonic pulse-echo technique. Phys. Rev. B 74, 184105 (2006).
Pecharsky, V. K. & Gschneidner, K. A. Jr Giant magnetocaloric effect in Gd5(Si2Ge2). Phys. Rev. Lett. 78, 4494–4497 (1997).
Katter, M., Zellmann, V., Reppel, G. W. & Uestuener, K. Magnetocaloric properties of La(Fe,Co,Si)13 bulk material prepared by powder metallurgy. IEEE Trans. Magn. 44, 3044 (2008).
Kanomata, T. et al. Magneto-volume effect of MnCo1−xGe (0 ≤ x ≤ 0.2). J. Magn. Magn. Mater. 140–144, 131–132 (1995).
Oliveira, F. et al. Process influences on the structure, piezoelectric, and gas-barrier properties of PVDF-TrFE copolymer. J. Polym. Sci. B 52, 496–506 (2014).
Shkuratov, S. I. et al. Depolarization mechanisms of PbZr0.52Ti0.48O3 and PbZr0.95Ti0.05O3 poled ferroelectrics under high strain rate loading. Appl. Phys. Lett. 104, 212901 (2014).
Mischenko, A. S., Zhang, Q., Scott, J. F., Whatmore, R. W. & Mathur, N. D. Giant electrocaloric effect in thin-film PbZr0.95Ti0.05O3. Science 311, 1270–1271 (2006).
Buttner, R. & Maslen, E. Structural parameters and electron difference density in BaTiO3. Acta Crystallogr. B 48, 764–769 (1992).
Moya, X. et al. Giant electrocaloric strength in single-crystal BaTiO3. Adv. Mater. 25, 1360–1365 (2013).
Wang, F. E., Buehler, W. J. & Pickart, S. J. Crystal structure and a unique “martensitic” transition of TiNi. J. Appl. Phys. 36, 3232–3239 (1965).
Bonnot, E., Romero, R., Mañosa, Ll., Vives, E. & Planes, A. Elastocaloric effect associated with the martensitic transition in shape-memory alloys. Phys. Rev. Lett. 100, 125901 (2008).
Nikitin, S. A. et al. Giant elastocaloric effect in FeRh alloy. Phys. Lett. A 171, 234–236 (1992).
Moore, M. J. & Kasper, J. S. Crystal structure of AgI at 3 kbar. J. Chem. Phys. 48, 2446 (1968).
Arroyo, M., Lopez-Manchado, M. & Herrero, B. Organo-montmorillonite as substitute of carbon black in natural rubber compounds. Polymer 44, 2447–2453 (2003).
Rose, H. A. & Vancamp, A. 2-Amino-2-methyl-1,2-propanediol. Anal. Chem. 28, 1790–1791 (1956).
Johnson, D. J., Ervin, J. S., Hanchak, M. & Hu, X. Graphite foam infused with pentaglycerine for solid-state thermal energy storage. J. Thermophys. Heat Transfer 29, 55–64 (2015).
Zhurov, V. V., Zhurova, E. A., Chen, Y. S. & Pinkerton, A. A. Accurate charge density data collection in under a day with a home X-ray source. J. Appl. Crystallogr. 38, 827–829 (2005).
Kendi, E. Molecular and crystal structure of tris(hydroxymethyl)aminomethane. Z. Kristallogr. 160, 139–143 (1982).
Tamarit, J. L. et al. Crystal data of the low-temperature solid form of 2-methyl-2-nitro-propanol. Powder Diffr. 9, 84–86 (1994).
Marr, H., Kruger, G. & Stewart, J. 2-Methyl-2-nitro-1, 3-propanediol. Acta Crystallogr. B 33, 2886–2887 (1977).
Acknowledgements
We acknowledge beam time awarded by J-PARC (proposals 2018AU1401 and 2018B0014), SPring-8 (proposals 2018B1095 and 2018A2061) and from ANSTO. B.L., Zhao Zhang, Zhe Zhang, W.R. and Zhidong Zhang were supported by the Hundred Talents Project of CAS and the National Natural Science Foundation of China (grants 11804346, 51671192 and 51531008). T.S. was supported by JSPS KAKENHI (grant number JP18K05032). S.L. and J.W. acknowledge financial support from the US National Science Foundation (grant number CBET-1708968) and the Florida State University through the Energy and Materials Initiative. H.W. acknowledges support from the National Natural Science Foundation of China (grant number 11874429) and the High-Level Talents Project of Hunan Province (grant number 2018RS3021). We also thank W. Zhang, A. Chen and H. Zeng of Setaram for testing the sample using a μDSC 7 EVO.
Reviewer information
Nature thanks Thomas Brueckel, Claudio Cazorla and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
B.L. and Zhidong Zhang conceived the idea. B.L., Y.K., T.K., S.O.-K., T.H. and K.N. performed the neutron scattering experiments at AMATERAS. T.H. calibrated the pressures at PLANET. S.-i.Y. collected the elastic data at SIKA. D.Y. and R.M. conducted the INS measurements at PELICAN. B.L., Y.C., S.I.K., K.O., S.K. and O.S. carried out synchrotron XRD measurements. K.L. analysed the XRD data. T.S. performed pressure-dependent DSC characterizations. H.W. calculated the vibrational spectra. J.W. and S.L. conducted the molecular dynamics simulations. Zhao Zhang, Zhe Zhang and W.R. carried out the in-house XRD and DSC measurements under ambient pressure for testing the samples. B.L. analysed neutron data and wrote the manuscript with discussion and input from all coauthors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Diffraction data and crystal structures of NPG.
a, Integrated intensity at −0.1 meV ≤ E ≤ 0.1 meV across Tt ≈ 314 K, obtained at AMATERAS. b, Elastic incoherent scattering intensity as a function of temperature at Q = 2.1 Å−1, measured at SIKA. The inset shows the crystal structure of the monoclinic phase. c, Synchrotron XRD patterns of NPG in the temperature region 273–353 K, obtained at BL02B2 with a wavelength of 0.9994 Å. d, Rietveld refinement of the data at 303 K. Expt, experimental data; Fit, simulated pattern; Diff, difference between experimental and simulated results; Bragg, position of Bragg peaks; Goodness, χ2. e, Temperature dependence of the lattice dimensions across the phase transition.
Extended Data Fig. 2 QENS analysis.
a–c, Energy resolution at different incident energies Ei. d, Activation energy (Ea) of the isotropic reorientational mode, obtained by fitting the temperature dependence of the experimental linewidth Γ. e, f, Simplified reorientational models. Brown, red and pink spheres represent carbon, oxygen and hydrogen atoms, respectively. Distances are given in ångströms. e, Triangles with side length of 1.57 Å indicate the configuration of hydrogen atoms in the methyl groups. The rotational radius of about 0.9 Å corresponds to the distance from the centre of the triangles to the hydrogen atoms. f, The distances between carbon atoms are labelled. The distance between the central carbon atom and C(CH3)–C(CH3), which links the two carbon atoms of the methyl groups, is about 0.84 Å, and that between the central carbon atom and C(CH2OH)–C(CH2OH), which links the two carbon atoms of the hydroxymethyl groups, is about 0.87 Å. These two distances are shown schematically on the right, and their average value is 0.855 Å. This isotropic reorientation model with a rotational radius of 0.855 Å describes a complex reorientational mode consisting of a free rotational reorientation of the whole molecule with respect to an axis perpendicular to C(CH3)–C(CH3) and C(CH2OH)–C(CH2OH), and an isotropic rotational reorientation of this axis.
Extended Data Fig. 3 INS data with higher Ei.
a, b, S(Q, E) obtained at AMATERAS with Ei = 23.72 meV (a) and Ei = 5.93 meV (b). c, d, Multi-component fit of S(Q, E) data at 2 Å−1 ≤ Q ≤ 3 Å−1 with Ei = 5.93 meV at 300 K (c) and 320 K (d). e, General density of state (GDOS), measured at PELICAN. The arrows indicate the peak positions of the mode around 12.7 meV.
Extended Data Fig. 4 QENS data.
a–e, Data obtained with Ei = 2.64 meV at 300 K for an empty cell (a), the pressure-transmitting medium (KBr; b, c) and the sample (d, e). It can be seen that the inelastic signal at Q = 1.3 Å−1 originates from Teflon, whereas the pressure-transmitting medium KBr does not contribute much to the background.
Extended Data Fig. 5 INS data.
a–e, Data obtained with Ei = 23.72 meV at 300 K for the empty cell (a), the pressure-transmitting medium (b, c) and the sample (d, e). The inelastic signals at about 2.7, 4.4 and 5.2 Å−1 are contributed by phonons from Teflon.
Extended Data Fig. 6 Additional pressure-dependence data.
a, S(Q, E) at Ei = 2.64 meV, 178 MPa and 325 K. b, S(Q, E) at Ei = 23.72 meV, 178 MPa and 325 K. c, Elastic incoherent scattering intensity integrated in 0.2 Å−1 ≤ Q ≤ 0.8 Å−1 with a ramping rate of 0.1 K min−1 at 178 MPa. The blue and red arrows indicate the cooling and warming processes, respectively. d, Entropy changes in NPG during heating for pressure changes from the ambient pressure (P0) to the applied pressure (P = 50, 80 and 100 MPa), obtained using μDSC 7 EVO at Setaram, France.
Extended Data Fig. 7 Theoretical modelling.
a, Atomic structure of the NPG molecule and its orientation, defined in three-dimensional space using angles α and θ in molecular dynamics simulations. Vector L points from the central carbon atom to a corner carbon atom of the molecule. b, Simulation results showing the distribution of the orientations of the molecules as a function of pressure at 340 K. It can be seen that a pressure of 100 MPa already effectively suppresses disorder. c, Full vibrational spectrum of NPG calculated by DFT. We note that there is a gap between 200 and 350 meV.
Rights and permissions
About this article
Cite this article
Li, B., Kawakita, Y., Ohira-Kawamura, S. et al. Colossal barocaloric effects in plastic crystals. Nature 567, 506–510 (2019). https://doi.org/10.1038/s41586-019-1042-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1042-5
This article is cited by
-
Low-k nano-dielectrics facilitate electric-field induced phase transition in high-k ferroelectric polymers for sustainable electrocaloric refrigeration
Nature Communications (2024)
-
Prediction and understanding of barocaloric effects in orientationally disordered materials from molecular dynamics simulations
npj Computational Materials (2024)
-
Magnetic Properties and Magnetocaloric Effect in Tb2FeCrO6 Double Perovskite Oxide
Journal of Electronic Materials (2024)
-
Scaling Laws of Elastocaloric Regenerators
Shape Memory and Superelasticity (2024)
-
Plastic Crystal Neopentyl Glycol/Multiwall Carbon Nanotubes Composites for Highly Efficient Barocaloric Refrigeration System
Journal of Thermal Science (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.