Abstract
The biochemical response to food intake must be precisely regulated. Because ingested sugars and fats can feed into many anabolic and catabolic pathways1, how our bodies handle nutrients depends on strategically positioned metabolic sensors that link the intrinsic nutritional value of a meal with intermediary metabolism. Here we describe a subset of immune cells—integrin β7+ natural gut intraepithelial T lymphocytes (natural IELs)—that is dispersed throughout the enterocyte layer of the small intestine and that modulates systemic metabolism. Integrin β7− mice that lack natural IELs are metabolically hyperactive and, when fed a high-fat and high-sugar diet, are resistant to obesity, hypercholesterolaemia, hypertension, diabetes and atherosclerosis. Furthermore, we show that protection from cardiovascular disease in the absence of natural IELs depends on the enteroendocrine-derived incretin GLP-12, which is normally controlled by IELs through expression of the GLP-1 receptor. In this metabolic control system, IELs modulate enteroendocrine activity by acting as gatekeepers that limit the bioavailability of GLP-1. Although the function of IELs may prove advantageous when food is scarce, present-day overabundance of diets high in fat and sugar renders this metabolic checkpoint detrimental to health.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Begg, D. P. & Woods, S. C. The endocrinology of food intake. Nat. Rev. Endocrinol. 9, 584–597 (2013).
Drucker, D. J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 24, 15–30 (2016).
Cerf-Bensussan, N., Bègue, B., Gagnon, J. & Meo, T. The human intraepithelial lymphocyte marker HML-1 is an integrin consisting of a β7 subunit associated with a distinctive α chain. Eur. J. Immunol. 22, 273–277 (1992).
Cheroutre, H., Lambolez, F. & Mucida, D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 11, 445–456 (2011).
Holzmann, B., McIntyre, B. W. & Weissman, I. L. Identification of a murine Peyer’s patch—specific lymphocyte homing receptor as an integrin molecule with an α chain homologous to human VLA-4α. Cell 56, 37–46 (1989).
Holzmann, B. & Weissman, I. L. Peyer’s patch-specific lymphocyte homing receptors consist of a VLA-4-like α chain associated with either of two integrin β chains, one of which is novel. EMBO J. 8, 1735–1741 (1989).
Parker, C. M. et al. A family of β7 integrins on human mucosal lymphocytes. Proc. Natl Acad. Sci. USA 89, 1924–1928 (1992).
Gorfu, G., Rivera-Nieves, J. & Ley, K. Role of β7 integrins in intestinal lymphocyte homing and retention. Curr. Mol. Med. 9, 836–850 (2009).
Buck, M. D., Sowell, R. T., Kaech, S. M. & Pearce, E. L. Metabolic instruction of immunity. Cell 169, 570–586 (2017).
Johnson, A. M. & Olefsky, J. M. The origins and drivers of insulin resistance. Cell 152, 673–684 (2013).
Lumeng, C. N. & Saltiel, A. R. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 121, 2111–2117 (2011).
Odegaard, J. I. & Chawla, A. The immune system as a sensor of the metabolic state. Immunity 38, 644–654 (2013).
Swirski, F. K. & Nahrendorf, M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339, 161–166 (2013).
Swirski, F. K. et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 117, 195–205 (2007).
Hilgendorf, I. & Swirski, F. K. Making a difference: monocyte heterogeneity in cardiovascular disease. Curr. Atheroscler. Rep. 14, 450–459 (2012).
Berlin, C. et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74, 185–195 (1993).
Wagner, N. et al. Critical role for β7 integrins in formation of the gut-associated lymphoid tissue. Nature 382, 366–370 (1996).
Schön, M. P. et al. Mucosal T lymphocyte numbers are selectively reduced in integrin αE (CD103)-deficient mice. J. Immunol. 162, 6641–6649 (1999).
Uehara, S., Grinberg, A., Farber, J. M. & Love, P. E. A role for CCR9 in T lymphocyte development and migration. J. Immunol. 168, 2811–2819 (2002).
Lycke, N. Y. & Bemark, M. The regulation of gut mucosal IgA B-cell responses: recent developments. Mucosal Immunol. 10, 1361–1374 (2017).
Fagarasan, S. & Honjo, T. Intestinal IgA synthesis: regulation of front-line body defences. Nat. Rev. Immunol. 3, 63–72 (2003).
Baggio, L. L. & Drucker, D. J. Biology of incretins: GLP-1 and GIP. Gastroenterology 132, 2131–2157 (2007).
Kahles, F. et al. GLP-1 secretion is increased by inflammatory stimuli in an IL-6-dependent manner, leading to hyperinsulinemia and blood glucose lowering. Diabetes 63, 3221–3229 (2014).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
Yusta, B. et al. GLP-1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP-1R. Diabetes 64, 2537–2549 (2015).
Lamont, B. J. et al. Pancreatic GLP-1 receptor activation is sufficient for incretin control of glucose metabolism in mice. J. Clin. Invest. 122, 388–402 (2012).
Arakawa, M. et al. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes 59, 1030–1037 (2010).
Nagashima, M. et al. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia 54, 2649–2659 (2011).
Kim, M. et al. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat. Med. 19, 567–575 (2013).
Acknowledgements
This work was supported by NIH grants R35 HL135752, R01 HL128264, P01 HL131478, the AHA EIA and the Patricia and Scott Eston MGH Research Scholar (to F.K.S.). S.H. was supported by an AHA Postdoctoral Award (16POST27250124); F.K. and W.C.P. by the German Research Foundation (DFG); S.R. was supported by a postdoctoral fellowship from the Swedish Research Council; M. Nairz was supported by a FWF Erwin Schroedinger Fellowship (J3486-B13); J.E.M. was supported by a NIH training grant (T32 AI118692); L.H. was supported by a Boehringer Ingelheim Fonds MD Fellowship; and D.J.D. was supported by a CIHR grant 154321, the Canada Research Chairs program and a BBDC-Novo Nordisk Chair in Incretin biology. We thank K. Joyes for copy-editing the manuscript.
Reviewer information
Nature thanks D. Mucida, F. Reimann and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
S.H. conceived the project, designed and performed experiments, analysed and interpreted data and made the figures; F.K. performed experiments, analysed and interpreted data and made the figures; S.R., M. Nairz, A.A., C.S.M., D.S., A.M.F., C.T.C., J.E.M., C.V., W.C.P., Y.I., G.R.W. and C.F.-H. performed experiments; R.W., P.L., C.F.-H., D.J.D. and M. Nahrendorf provided intellectual input; R.W., C.F.-H., D.J.D. and M. Nahrendorf provided materials; F.K.S. conceived the project, designed experiments, interpreted data and wrote the manuscript. All authors edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The General Hospital Corporation has filed a US patent application 62/771,668 with the US Patent and Trademark office entitled ‘Targeting intraepithelial leukocytes for treatment of cardiometabolic diseases’, which names F.K.S. and S.H. as inventors. D.J.D. has served as an advisor or consultant to Intarcia, Forkhead Biopharmaceuticals Inc., Kallyope Inc., Merck Research Laboratories, Pfizer Inc., Novo Nordisk Inc. and Zafgen Inc. Mount Sinai receives funding from GSK, Merck and Novo Nordisk for GLP-1-related studies in the Drucker laboratory.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Effects of integrin β7 deficiency on metabolism.
a, Metabolic cage measurements of activity in wild-type (WT) and β7−/− mice. n = 4 mice per group. b, O2 consumption and CO2 production. n = 5 wild-type mice; n = 4 β7−/− mice. c, Respiratory exchange rate (RER) by CLAMS in wild-type and β7−/− mice that were fed a chow diet. n = 5 wild-type mice; n = 4 β7−/− mice; *P < 0.05, two-tailed Mann–Whitney U-test. d, Overnight-fasted wild-type and β7−/− mice were administered [18F]FDG. The radioactivity in indicated organs was measured as the percentage injected dose per gram tissue weight (%ID/g). n = 6 wild-type mice; n = 5 β7−/− mice; *P < 0.05, Student’s t-test. e, Wild-type and β7−/− mice were housed in thermoneutral (TN) incubators for three days and then subjected to the intraperitoneal (IP) glucose-tolerance test. n = 5 mice per group; **P < 0.01, two-tailed Mann–Whitney U-test. f, Wild-type and β7−/− mice were treated with antibiotic cocktails in drinking water for 4 weeks and then subjected to the glucose-tolerance test. n = 4 mice per group; *P < 0.05, two-tailed Mann–Whitney U-test. g, Eight-week-old wild-type and β7−/− mice were cohoused at a ratio of 1:1 for 4 weeks and then subjected to the glucose-tolerance test. n = 7 mice per group; *P < 0.05, two-tailed Mann–Whitney U-test. h, Fat absorption was analysed using a fat-tolerance test in the presence of P407. n = 9 wild-type mice; n = 6 β7−/− mice. i, For assessment of permeability, mice were gavaged with FITC–dextran and fluorescence was measured in the plasma 4 h later. A wild-type mouse subjected to a dextran sulfate sodium (DSS)-induced colitis model was used as a positive control for increased gut permeability. n = 8 wild-type mice; n = 7 β7−/− mice; P = 0.17, two-tailed Mann–Whitney U-test. Data are mean ± s.e.m.
Extended Data Fig. 2 Effects of integrin β7 deficiency on obesity, cholesterolaemia and atherosclerosis.
a, Representative flow cytometry plots gated on CD45+ non-T and B (TB) leukocytes and quantification of Ly-6Chigh monocytes, neutrophils and macrophages (Mϕ) in iWAT of wild-type and β7−/− mice fed a HFSSD for 5 months. n = 5 wild-type mice; n = 4 β7−/− mice; *P < 0.05, two-tailed Mann–Whitney U-test. b, Representative flow cytometry plots and quantification of Ly-6Chigh monocytes, neutrophils and macrophages in pWAT of wild-type and β7−/− mice fed a HFSSD for 5 months. n = 5 wild-type mice; n = 4 β7−/− mice; *P < 0.05, two-tailed Mann–Whitney U-test. c, Plasma glucose levels measured in overnight-fasted animals fed a HFSSD for 5 months. n = 10 wild-type mice; n = 7 β7−/− mice; ***P < 0.001, two-tailed Mann–Whitney U-test. d, Insulin levels measured in overnight-fasted animals fed a HFSSD for 5 months. n = 9 wild-type mice; n = 8 β7−/− mice; *P < 0.05, two-tailed Mann–Whitney U-test. e, Fasting plasma total cholesterol levels of mice fed a chow diet. n = 6 mice per group. f, Body weight changes during a 14-week HCD diet of bmβ7+/+Ldlr−/− and bmβ7−/−Ldlr−/− mice. n = 5 mice per group. g, Faecal cholesterol levels in bmβ7+/+Ldlr−/− and bmβ7−/−Ldlr−/− mice after a 14-week HCD diet. n = 5 mice per group; P = 0.09, two-tailed unpaired Student’s t-test. h–j, Representative images and histological quantification of macrophages (h), collagen content and necrotic core size (i) and smooth muscle cell content (j) of bmβ7+/+Ldlr−/− and bmβ7−/−Ldlr−/− mice after 14 weeks on a HCD. n = 5 mice per group; **P < 0.01, ***P < 0.001, two-tailed unpaired Student’s t-test. Data are mean ± s.e.m.
Extended Data Fig. 3 Effects of integrin β7 deficiency on myeloid cells and glucose tolerance.
a, Ldlr−/− mice were lethally irradiated and reconstituted with bone marrow mixtures of wild-type and β7−/− mice (1:1) and fed a chow diet or HCD for 14 weeks. b, The aortic leukocytes from different origins were analysed by flow cytometry. n = 4 mice for both HCD recipients and chow recipients. c, Ly-6Chigh and Ly-6Clow monocyte numbers in blood (n = 3 wild-type mice; n = 5 β7−/− mice), bone marrow (n = 3 wild-type mice; n = 5 β7−/− mice) and spleen (n = 6 mice per group) of wild-type and β7−/− mice fed a chow diet. d, bmβ7+/+Ldlr−/− and bmβ7−/−Ldlr−/− mice fed a HCD were subjected to an intraperitoneal glucose-tolerance test. n = 5 mice per group; *P < 0.05, two-tailed Mann–Whitney U-test. Data are mean ± s.e.m.
Extended Data Fig. 4 Effects of genetic deficiency and blocking of integrin β7 on atherosclerosis.
a, Body weights, cumulative food intake and energy expenditure were measured in Ldlr−/− mice and β7−/−Ldlr−/− mice. n = 4 mice per group. b, Ldlr−/− mice and β7−/−Ldlr−/− mice were fed a HCD for 14 weeks. Plasma cholesterol levels were determined in overnight-fasted mice. n = 7 Ldlr−/− mice; n = 5 β7−/−Ldlr−/− mice; **P < 0.01, two-tailed Mann–Whitney U-test. c, Representative oil-red O images and quantification of plaque size in the aortic roots. n = 7 Ldlr−/− mice; n = 5 β7−/−Ldlr−/− mice; *P < 0.05, two-tailed Mann–Whitney U-test. d, Quantification of Ly-6Chigh monocytes, neutrophils and macrophages in plaques. n = 7 mice per group. *P < 0.05, **P < 0.01, two-tailed Mann–Whitney U-test. e, Ldlr−/− mice that were fed a HCD were treated with anti-integrin β7 antibodies or IgG isotype control (500 μg per mouse per week) for 14 weeks. Mice were subjected to a glucose-tolerance test after 8 weeks on the HCD. n = 6 mice per group; **P < 0.01, two-tailed Mann–Whitney U-test. f, Representative images of oil-red O-stained aortic cross-sections and quantification of plaque size in the aortic roots after 14 weeks on a HCD. n = 5 mice treated with IgG; n = 6 mice treated with anti-integrin β7 antibody; *P < 0.05, **P < 0.01, two-tailed Mann–Whitney U-test. Data are mean ± s.e.m.
Extended Data Fig. 5 Integrin β7 guides leukocytes to gut intraepithelium.
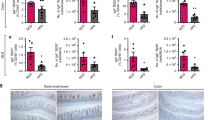
a, Top, representative histology staining for CD3 in small intestines of wild-type and β7−/− mice. Bottom, quantification of CD3+ cells in each villus (more than 15 villi were counted for each mouse). ***P < 0.001, two-way ANOVA. b, Schematic of the competitive transfer experiments. Mice (CD45.2+) were lethally irradiated and transplanted with a 1:1 ratio mix of GFP+β7−/− bone marrow cells and CD45.1+ wild-type bone marrow cells. The chimerism in different tissues was normalized to the ratio in blood. n = 5 mice. c, Representative flow cytometry plots and quantification of B cells and myeloid cells in mice depicted in b. n = 5 biologically independent recipients. d, Quantification of γδT cells in the liver. n = 3 wild-type mice; n = 4 β7−/− mice. WBC, white blood cells. e, Quantification of γδT cells in the pancreas. n = 4 wild-type mice; n = 5 β7−/− mice. f, Wild-type mice were lethally irradiated and reconstituted with bone marrow cell mixtures of β7−/− and wild-type (β7−/−:wild-type cells, 1:1 ratio) or β7−/− and indicated knockout mice (β7−/−:knockout cells, 1:1 ratio). The indicated mixed chimaeras that specifically lack intestinal B cells (β7−/−μMT) or myeloid cells (β7−/−Ccr2−/−) were subjected to oral glucose-tolerance tests and the AUCs are shown. n = 5 mice per group for β7−/− wild-type and β7−/−μMT mice; n = 4 mice per group for β7−/− wild-type and β7−/−Ccr2−/− mice. Data are mean ± s.e.m.
Extended Data Fig. 6 B cells are dispensable for the altered metabolic phenotypes in integrin β7-deficient mice.
Ldlr−/− mice were lethally irradiated and reconstituted with bone marrow cell mixtures of β7−/− and wild-type (β7−/− WT, 1:1 ratio) or β7−/− and μMT (β7−/−μMT, 1:1 ratio). The reconstituted mixed chimaeras were fed a HCD for 14 weeks. a, IgA levels in the gut flush (n = 5 β7−/− wild-type mice; n = 4 β7−/−μMT) and plasma (n = 5 β7−/− wild-type mice; n = 3 β7−/−μMT mice). **P < 0.01, two-tailed Mann–Whitney U-test. b, Number of IgD+ B cells in Peyer’s patches (PP) and IgA+ B cells and IgD+ B cells in lamina propria (LP) as determined by flow cytometry. n = 5 β7−/− wild-type mice; n = 3 β7−/−μMT mice; *P < 0.05, two-tailed Mann–Whitney U-test. c, Glucose-tolerance test in HCD-fed mixed chimaeras. n = 5 β7−/− wild-type mice; n = 3 β7−/−μMT mice. d, Plasma cholesterol levels in overnight-fasted mice. n = 5 β7−/− wild-type mice; n = 4 β7−/−μMT mice. e, Representative images and quantification of oil-red O staining in aorta root sections of bmβ7−/− wild-type Ldlr−/− mice and bmβ7−/−μMT Ldlr−/− mice that were fed a HCD for 14 weeks. n = 5 bmβ7−/− wild-type Ldlr−/− mice; n = 4 bmβ7−/−μMT Ldlr−/− mice. Data are mean ± s.e.m.
Extended Data Fig. 7 Integrin β7 deficiency and GLP-1.
a, Plasma total GLP-1 levels after overnight fasting and 15 min after oral glucose load (2 g per kg body weight) in wild-type and β7−/− mice that were fed a chow diet. Total GLP-1 fasting: n = 7 mice per group; total GLP-1 after oral glucose-tolerance test (OGTT) 15 min: n = 7 wild-type mice; n = 6 β7−/− mice. b, Plasma total GLP-1 levels after 5 months of a HFSSD. Total GLP-1 fasting: n = 7 mice per group; total GLP-1 oral glucose-tolerance test 15 min: 6 mice per group. c, Representative flow cytometry plots of small-intestinal IELs from wild-type and β7−/− mice. d, Glp1r mRNA levels in sorted different IEL subsets from wild-type and β7−/− mice. n = 4 wild-type mice; n = 5 β7−/− mice. e, Wild-type mice were lethally irradiated and transplanted with a 1:1 bone marrow mixture of wild-type and GFP+ or Glp1r−/− and GFP+. The chimerism in different tissues was analysed by comparing the percentage of GFP+ leukocytes normalized to wild-type GFP+ blood leukocytes. n = 4 mice per group. Data are mean ± s.e.m. *P < 0.05, ***P < 0.001. All P values from two-tailed unpaired Student’s t-tests.
Extended Data Fig. 8 Effects of Glp1r deficiency on IELs and atherosclerosis.
a, Quantification of small-intestinal IEL subpopulations in bmGlp1r+/+β7−/−Ldlr−/− and bmGlp1r−/−/β7−/−Ldlr−/− mice. n = 5 mice per group, mean ± s.e.m. b, Glp1r mRNA expression of sorted IEL subpopulations. n = 4 biologically independent bmGlp1r+/+β7−/− and n = 5 biologically independent bmGlp1r−/−β7−/− mice; two-tailed unpaired Student’s t-test. c, Glp1r mRNA expression in the liver (n = 5 bmGlp1r+/+β7−/− and n = 4 bmGlp1r−/−β7−/− mice), heart and lung tissue (n = 5 mice per group). d, Quantification of γδ T cells from the liver (n = 5 mice per group) and pancreas (n = 5 bmGlp1r+/+β7−/−Ldlr−/− mice; n = 4 bmGlp1r−/−β7−/−Ldlr−/− mice). e, Glp1r mRNA expression of sorted γδ T cells from pancreas, liver and small-intestinal IELs. n = 3 mice per group; two-tailed unpaired Student’s t-test. f, Oral glucose-tolerance test. n = 4 bmGlp1r+/+ mice; n = 3 bmGlp1r−/− mice. g, GLP-1 levels after overnight fasting (n = 4 mice per group) or oral glucose challenge (n = 4 bmGlp1r+/+ mice; n = 3 bmGlp1r−/− mice). h, Oral glucose-tolerance test in bmGlp1r+/+β7−/− and bmGlp1r−/−β7−/− mice. n = 5 mice per group; two-tailed Mann–Whitney U-test. i, GLP-1 levels after overnight fasting or oral glucose challenge in bmGlp1r+/+β7−/− and bmGlp1r−/−β7−/− mice. n = 5 mice per group; two-tailed unpaired Student’s t-test. j, Ldlr−/− mice were treated with the GLP-1 receptor agonist exendin-4 (Ex-4) at a dose of 100 μg per kg per day via osmotic minipumps (PBS was used as control). After 8 weeks on a HCD, mice were euthanized to enable the quantification of atherosclerotic lesions. Representative images of oil-red O-stained aortas and quantification of plaque size. k, Quantification of blood Ly-6Chigh monocytes, Ly-6Clow monocytes and neutrophils. n = 8 Ldlr−/− mice treated with exendin-4; n = 6 Ldlr−/− mice treated with PBS; two-tailed unpaired Student’s t-test. Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001.
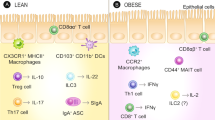
Extended Data Fig. 9 Gut intraepithelial Glp1rhigh IELs regulate the bioavailability of GLP-1.
a, Immunohistochemistry and quantification of GLP-1-producing L-cells in whole ileum preparations of 6 wild-type and 5 β7−/− mice. b, Small-intestinal IEL mixtures were incubated with the fluorescently (Cys40SeTau647) labelled GLP-1R agonist exendin-4 and the capacity of agonist binding by the different subsets—natural IELs (Glp1rhigh), induced IELs (Glp1rlow) and non-T cells—was analysed by flow cytometry. Sorted Glp1rhigh and Glp1rlow cells were also incubated with recombinant GLP-1 and the remaining supernatant GLP-1 was plotted against the relative Glp1r mRNA levels of the cells. c, GLP-1-producing GLUTag cells were co-cultured with sorted natural (Glp1rhigh) or induced (Glp1rlow) IELs. After 24 h, the concentration of GLP-1 in the supernatant was measured. n = 5 biologically independent samples for Glp1rhigh IELs and n = 4 biologically independent samples for Glp1rlow IELs. d, Left, GLUTag cells were co-cultured with sorted Glp1rhigh IELs in the presence of exendin-4 (100 nM) or control. n = 3 independent biologically samples per group. Right, GLUTag cells were stimulated with exendin-4 (100 nM) or control. n = 4 independent biological samples per group. After 24 h, the concentration of GLP-1 in the supernatant was measured. e, Sorted Glp1rhigh IELs were incubated with exendin-4 (100 nM) or control. After 24 h, samples were centrifuged and supernatants were transferred to ex vivo ileum fractions of wild-type mice. GLP-1 levels were determined 24 h later from ex vivo supernatants. n = 10 biologically independent mice per group. f, Whole gut preparations of wild-type or β7−/− mice were treated with or without the GLP-1 receptor antagonist exendin-9 (100 nM). After 24 h, the concentration of GLP-1 in the supernatant was measured. n = 5 biologically independent samples for wild-type or β7−/− mice without exendin-9; n = 4 biologically independent samples for wild-type mice with exendin-9. Data are mean ± s.e.m. *P < 0.05, **P < 0.01. All P values from two-tailed unpaired Student’s t-test.
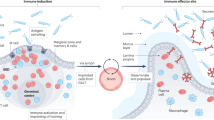
Extended Data Fig. 10 Model.
In this study, we propose that integrin β7-dependent Glp1rhigh IELs that reside in the small intestine modulate dietary metabolism in mice by restricting the bioavailability of GLP-1. The illustration was modified from Servier Medical Art (http://smart.servier.com/), licensed under a Creative Common Attribution 3.0 Generic License.
Supplementary information
Source data
Rights and permissions
About this article
Cite this article
He, S., Kahles, F., Rattik, S. et al. Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease. Nature 566, 115–119 (2019). https://doi.org/10.1038/s41586-018-0849-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0849-9
This article is cited by
-
Beyond the pancreas: contrasting cardiometabolic actions of GIP and GLP1
Nature Reviews Endocrinology (2023)
-
The therapeutic value of bifidobacteria in cardiovascular disease
npj Biofilms and Microbiomes (2023)
-
GLP-1 Receptor Agonists in Obese Patients with Inflammatory Bowel Disease: from Molecular Mechanisms to Clinical Considerations and Practical Recommendations for Safe and Effective Use
Current Obesity Reports (2023)
-
The gut hormone glucose-dependent insulinotropic polypeptide is downregulated in response to myocardial injury
Cardiovascular Diabetology (2022)
-
How the immune system shapes atherosclerosis: roles of innate and adaptive immunity
Nature Reviews Immunology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.