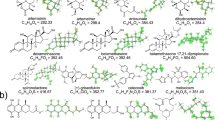
Abstract
Metal–organic frameworks (MOFs) are crystalline synthetic porous materials formed by binding organic linkers to metal nodes: they can be either rigid1,2 or flexible3. Zeolites and rigid MOFs have widespread applications in sorption, separation and catalysis that arise from their ability to control the arrangement and chemistry of guest molecules in their pores via the shape and functionality of their internal surface, defined by their chemistry and structure4,5. Their structures correspond to an energy landscape with a single, albeit highly functional, energy minimum. By contrast, proteins function by navigating between multiple metastable structures using bond rotations of the polypeptide6,7, where each structure lies in one of the minima of a conformational energy landscape and can be selected according to the chemistry of the molecules that interact with the protein. These structural changes are realized through the mechanisms of conformational selection (where a higher-energy minimum characteristic of the protein is stabilized by small-molecule binding) and induced fit (where a small molecule imposes a structure on the protein that is not a minimum in the absence of that molecule)8. Here we show that rotation about covalent bonds in a peptide linker can change a flexible MOF to afford nine distinct crystal structures, revealing a conformational energy landscape that is characterized by multiple structural minima. The uptake of small-molecule guests by the MOF can be chemically triggered by inducing peptide conformational change. This change transforms the material from a minimum on the landscape that is inactive for guest sorption to an active one. Chemical control of the conformation of a flexible organic linker offers a route to modifying the pore geometry and internal surface chemistry and thus the function of open-framework materials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets supporting the findings of this study are available from the University of Liverpool, and can be found at http://datacat.liverpool.ac.uk/id/eprint/589.
References
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal-organic frameworks. Science 341, 1230444 (2013).
Howarth, A. J. et al. Chemical, thermal and mechanical stabilities of metal–organic frameworks. Nat. Rev. Mater. 1, 15018 (2016).
Kitagawa, S., Kitaura, R. & Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 43, 2334–2375 (2004).
Moliner, M., Martínez, C. & Corma, A. Multipore zeolites: synthesis and catalytic applications. Angew. Chem. Int. Ed. 54, 3560–3579 (2015).
Kaskel, S. in The Chemistry of Metal–Organic Frameworks 1–3 (Wiley-VCH, Weinheim, Germany, 2016).
Beck, D. A. C., Alonso, D. O. V., Inoyama, D. & Daggett, V. The intrinsic conformational propensities of the 20 naturally occurring amino acids and reflection of these propensities in proteins. Proc. Natl Acad. Sci. USA 105, 12259–12264 (2008).
Swain, J. F. & Gierasch, L. M. The changing landscape of protein allostery. Curr. Opin. Struct. Biol. 16, 102–108 (2006).
Csermely, P., Palotai, R. & Nussinov, R. Induced fit, conformational selection and independent dynamic segments: an extended view of binding events. Trends Biochem. Sci. 35, 539–546 (2010).
Schneemann, A. et al. Flexible metal-organic frameworks. Chem. Soc. Rev. 43, 6062–6096 (2014).
Horike, S., Shimomura, S. & Kitagawa, S. Soft porous crystals. Nat. Chem. 1, 695–704 (2009).
Loiseau, T. et al. A rationale for the large breathing of the porous aluminum terephthalate (MIL-53) upon hydration. Chem. Eur. J. 10, 1373–1382 (2004).
Nouar, F. et al. Tuning the breathing behaviour of MIL-53 by cation mixing. Chem. Commun. 48, 10237–10239 (2012).
Serre, C. et al. Role of solvent-host interactions that lead to very large swelling of hybrid frameworks. Science 315, 1828–1831 (2007).
Maji, T. K., Matsuda, R. & Kitagawa, S. A flexible interpenetrating coordination framework with a bimodal porous functionality. Nat. Mater. 6, 142–148 (2007).
Mason, J. A. et al. Methane storage in flexible metal-organic frameworks with intrinsic thermal management. Nature 527, 357–361 (2015).
Freye, S. et al. Allosteric binding of halide anions by a new dimeric interpenetrated coordination cage. Angew. Chem. Int. Ed. 51, 2191–2194 (2012).
Freye, S. et al. Template control over dimerization and guest selectivity of interpenetrated coordination cages. J. Am. Chem. Soc. 135, 8476–8479 (2013).
Löffler, S. et al. Triggered exchange of anionic for neutral guests inside a cationic coordination cage. J. Am. Chem. Soc. 137, 1060–1063 (2015).
Lin, Z.-J., Lü, J., Hong, M. & Cao, R. Metal-organic frameworks based on flexible ligands (FL-MOFs): structures and applications. Chem. Soc. Rev. 43, 5867–5895 (2014).
Colodrero, R. M. P. et al. Multifunctional lanthanum tetraphosphonates: flexible, ultramicroporous and proton-conducting hybrid frameworks. Dalton Trans. 41, 4045–4051 (2012).
Rouschmeyer, P. et al. A flexible fluorescent Zr carboxylate metal-organic framework for the detection of electron-rich molecules in solution. Inorg. Chem. 56, 8423–8429 (2017).
Yeung, H.-M. H., Kosa, M., Parrinello, M. & Cheetham, A. K. Chiral, racemic, and meso-lithium tartrate framework polymorphs: a detailed structural analysis. Cryst. Growth Des. 13, 3705–3715 (2013).
Motkuri, R. K. et al. Role of hydrocarbons in pore expansion and contraction of a flexible metal-organic framework. Chem. Commun. 47, 7077–7079 (2011).
Murdock, C. R., Lu, Z. & Jenkins, D. M. Effects of solvation on the framework of a breathing copper MOF employing a semirigid linker. Inorg. Chem. 52, 2182–2187 (2013).
Haddad, J., Whitehead, G. F. S., Katsoulidis, A. P. & Rosseinsky, M. J. In-MOFs based on amide functionalised flexible linkers. Faraday Discuss. 201, 327–335 (2017).
Rabone, J. et al. An adaptable peptide-based porous material. Science 329, 1053–1057 (2010).
Martí-Gastaldo, C. et al. Side-chain control of porosity closure in single- and multiple-peptide-based porous materials by cooperative folding. Nat. Chem. 6, 343–351 (2014).
Katsoulidis, A. P. et al. Guest-adaptable and water-stable peptide-based porous materials by imidazolate side chain control. Angew. Chem. Int. Ed. 53, 193–198 (2014).
Mu, Y., Nguyen, P. H. & Stock, G. Energy landscape of a small peptide revealed by dihedral angle principal component analysis. Proteins 58, 45–52 (2005).
Naganathan, A. N., Sanchez-Ruiz, J. M. & Muñoz, V. Direct measurement of barrier heights in protein folding. J. Am. Chem. Soc. 127, 17970–17971 (2005).
Sheppard, D., Terrell, R. & Henkelman, G. Optimization methods for finding minimum energy paths. J. Chem. Phys. 128, 134106 (2008).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Dion, M., Rydberg, H., Schröder, E., Langreth, D. C. & Lundqvist, B. I. van der Waals density functional for general geometries. Phys. Rev. Lett. 92, 246401 (2004).
Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement number 692685) and from the Engineering and Physical Sciences Research Council (EPSRC; grant EP/J008834). We acknowledge the High Performance Computing (HPC) Materials Chemistry Consortium for providing access to UK’s national supercomputer ARCHER under EPSRC grant EP/L000202. We thank the Diamond Light Source for provision of beamtime on the I11 and I19 beamlines, and the X-ray crystallography facility of the School of Chemistry at the University of Manchester for the use of their Rigaku Oxford diffraction FR-X instrument to collect two of the single-crystal diffraction datasets.
Author information
Authors and Affiliations
Contributions
A.P.K., D.A. and M.J.R. conceived the research and wrote the first draft of the paper. A.P.K. and M.J.R. designed the experimental approach. A.P.K. synthesized the materials and carried out the adsorption experiments. D.J.A. provided input on materials synthesis and characterization. D.A., M.S.D., G.R.D. and N.G.B. designed the computational approach. D.A. performed the theoretical simulations. G.F.S.W. and E.J.C. performed the structural analyses. All the authors discussed the results and contributed to writing the manuscript. M.J.R. directed the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Synthesis and crystal structure of ZnGGH-1⋅(DMF-H2O).
a, Schematic representation of ZnGGH-1⋅(DMF-H2O) synthesis from glycine–glycine–l-histidine and Zn(OAc)2. The tripeptide is deprotonated at the C-terminal acid and the imidazole side chain. The resulting anion acts as a tetratopic linker and coordinates to four Zn2+ cations, thus forming a three-dimensional (4,4)-connected net. b, Top, structural representation of ZnGGH-1⋅(DMF-H2O) viewed along the b axis reveals the parallelogram-shaped one-dimensional pores. Centre, the rigid layers of the framework are composed of the histidine residue. Each histidine links three Zn2+ cations; two of them are bridged by the imidazolate side chain, and the third is bound to the carboxylate group. Bottom, ZnGGH-1⋅(DMF-H2O) viewed along the a axis shows the undulating shape of the rigid layers, which are interconnected by the flexible glycine–glycine chains.
Extended Data Fig. 2 The structural transformations of ZnGGH and the experimental workflow for triggered guest adsorption.
a, Eight distinct crystal structures are produced by exchanging the molecules that occupy the pores of as-made ZnGGH-1⋅(DMF-H2O). b, Experimental procedure for the adsorption of dioxane by ZnGGH-2 with (left) and without (right) triggering by DMF. Adding DMF during the dioxane-adsorption experiment produces a structural transformation and allows dioxane to access the ZnGGH framework. In the absence of DMF, dioxane does not access the pores of ZnGGH-2. PXRD and 1H-NMR data are shown in Fig. 2. The small amount of dioxane measured in the non-triggered adsorption is assigned to surface species that are not removed during the required work-up procedure.
Extended Data Fig. 3 Structure and linker conformation of ZnGGH-2⋅(DMSO).
ZnGGH-2⋅(DMSO) is produced by treating ZnGGH-1⋅(DMF-H2O) with pure DMSO. This reaction is reversible: ZnGGH-2⋅(DMSO) is transformed to ZnGGH-1⋅(DMF-H2O) after treatment with a DMF/H2O 3/1 (v/v) mixture. The linker in ZnGGH-2 adopts a twisted conformation, distinct from the straight conformation adopted in ZnGGH-1, which causes the pore window to shrink from 4.9 Å to 4.5 Å. The twisted conformation directs the glycine–glycine amide group inside the pore, where it forms a hydrogen bond with DMSO via the NH group of the central glycine. By contrast, the glycine–glycine amide group in ZnGGH-1⋅(DMF-H2O) is directed along the pore direction and does not form a hydrogen bond with the DMF molecule. From top to bottom are shown parts of the structures at increasing magnification.
Extended Data Fig. 4 Structure, linker conformation and DMF orientation in ZnGGH-3⋅(DMF) and ZnGGH-1⋅(DMF-H2O).
ZnGGH-3⋅(DMF) is produced by treating ZnGGH-1⋅(DMF-H2O) with pure DMF. This reaction is reversible: ZnGGH-3⋅(DMF) is transformed back to ZnGGH-1⋅(DMF-H2O) after treatment with a DMF/H2O 3/1 (v/v) mixture. In both structures, the DMF molecule is hydrogen-bonded to the same proton of the terminal NH2, but its orientation changes by about 90° between structures 1 and 3. This change in DMF orientation is associated with repositioning of the terminal glycine between the two structures through conformational change of the tripeptide.
Extended Data Fig. 5 Structure and linker conformation of collapsed ZnGGH-9.
The collapsed structure ZnGGH-9 is produced by removing the solvents from ZnGGH-1⋅(DMF-H2O). In ZnGGH-9, the linker adopts two very similar folded conformations. These conformational changes are realized by the torsions φ2 of histidine and ψ of the central glycine.
Extended Data Fig. 6 Three clusters of conformations—straight, twisted and folded—are observed in ZnGGH structures, with the ‘straight’ cluster divided into four subgroups.
a, We applied dPCA to the linker conformations of the nine experimentally observed ZnGGH structures, grouping them into three clusters: straight, twisted and folded (Fig. 4a). As identified by dPCA, the main variance in linker conformation comes from the torsions φ2 of histidine and ω of Gly–His (contributing to the first principal component, PC1) as well as torsions φ1 and ψ of the central glycine (contributing to the second principal component, PC2). b, The straight cluster is the most populated, containing six different conformations. dPCA applied to these six conformations shows that they further separate into four subgroups (Fig. 4b). The main contribution to the principal components is from the torsions φ1 and ψ of the central glycine.
Extended Data Fig. 7 Comparison of the structures of ZnGGH-5⋅(H2O-DMF), ZnGGH-1⋅(DMF-H2O) and ZnGGH-3⋅(DMF).
ZnGGH-5⋅(H2O-DMF) (centre) is produced by treating ZnGGH-1⋅(DMF-H2O) with H2O/DMF = 5/3 (v/v). This structure combines the hydrogen bonding seen in ZnGGH-3⋅(DMF) (left) and ZnGGH-1⋅(DMF-H2O) (right). Although the linker conformations in these three structures belong to the same cluster (straight), the diversity between them (Extended Data Fig. 7b) is enough to produce different sets of hydrogen bonds, demonstrating the variety of structural subgroups shown by the second dPCA in Fig. 4b. ZnGGH-5⋅(H2O-DMF) has the maximum number of three possible intraframework hydrogen bonds (see Supplementary Table 4). There are two hydrogen bonds between the flexible glycine–glycine chain of the linker: one between glycine–glycine amide groups (green shading) is characteristic of ZnGGH-3, and another between the terminal NH2 and the glycine–histidine amide group (yellow shading) is characteristic of ZnGGH-1. The hydrogen bond between the histidine carboxylate and glycine–histidine amide (pink shading), in the rigid part of the framework, is present in every ZnGGH structure.
Extended Data Fig. 8 Comparison of hydrogen-bond patterns between ZnGGH-6⋅(F) and ZnGGH-7⋅(FA).
The furfural (F) and furfuryl alcohol (FA) guests produce two distinct structures, ZnGGH-6⋅(F) and ZnGGH-7⋅(FA) respectively. Although these structures feature peptide conformations that place them in the ‘straight linker’ cluster (Fig. 4a), they present different hydrogen-bond patterns, placing them in distinct subgroups in Fig. 4b. ZnGGH-6⋅(F) exhibits the same three intraframework hydrogen bonds as ZnGGH-5⋅(H2O-DMF), and the carbonyl group of furfural forms a hydrogen bond with the terminal NH2. By contrast, in ZnGGH-7⋅(FA), the hydroxyl of furfuryl alcohol is hydrogen-bonded to the glycine–glycine amide group and to the terminal NH2, while the flexible Gly–Gly chain of the linker has been rotated towards the guest to form these interactions, thereby disrupting two of the three intraframework hydrogen bonds observed in ZnGGH-6⋅(F): one between the Gly–Gly amide groups (green circle) and one between the terminal NH2 and the Gly–His amide group (purple circle). The only intraframework hydrogen bond that is present in ZnGGH-7⋅(FA) is between the histidine carboxylate and the Gly–His amide (black circle).
Extended Data Fig. 9 Structure and linker conformations of ZnGGH-4⋅(H2O).
ZnGGH-4⋅(H2O) is produced by treating ZnGGH-1⋅(DMF-H2O) with H2O. In ZnGGH-4⋅(H2O), the linker adopts two conformations. Conformation I (pink) is a straight conformation similar to that found in ZnGGH-1⋅(DMF-H2O), and conformation II (green) is a twisted conformation similar to that found in ZnGGH-2⋅(DMSO).
Supplementary information
Supplementary information
This file contains Supplementary Notes 1-7, Supplementary Figures S1-S60 and Supplementary Tables S1-S20.
Rights and permissions
About this article
Cite this article
Katsoulidis, A.P., Antypov, D., Whitehead, G.F.S. et al. Chemical control of structure and guest uptake by a conformationally mobile porous material. Nature 565, 213–217 (2019). https://doi.org/10.1038/s41586-018-0820-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0820-9
This article is cited by
-
Interaction-selective molecular sieving adsorbent for direct separation of ethylene from senary C2-C4 olefin/paraffin mixture
Nature Communications (2024)
-
Diffusion-rate sieving of propylene and propane mixtures in a cooperatively dynamic porous crystal
Nature Communications (2024)
-
Supramolecular polynuclear clusters sustained cubic hydrogen bonded frameworks with octahedral cages for reversible photochromism
Nature Communications (2024)
-
Light-driven anisotropy of 2D metal-organic framework single crystal for repeatable optical modulation
Communications Materials (2024)
-
Enhanced carbon capture with motif-rich amino acid loaded defective robust metal-organic frameworks
Nano Research (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.