Abstract
Starvation poses a fundamental challenge to cell survival. Whereas the role of autophagy in promoting energy homeostasis in this setting has been extensively characterized1, other mechanisms are less well understood. Here we reveal that glyceraldehyde 3-phosphate dehydrogenase (GAPDH) inhibits coat protein I (COPI) transport by targeting a GTPase-activating protein (GAP) towards ADP-ribosylation factor 1 (ARF1) to suppress COPI vesicle fission. GAPDH inhibits multiple other transport pathways, also by targeting ARF GAPs. Further characterization suggests that this broad inhibition is activated by the cell during starvation to reduce energy consumption. These findings reveal a remarkable level of coordination among the intracellular transport pathways that underlies a critical mechanism of cellular energy homeostasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability.
The data that support the findings of this study are available from the corresponding author upon reasonable request. The following figures have associated raw data: Figs. 1a, b, 2a–g, 3a–j, n–q, 4c–e, g–p; Extended Data Figs. 1c, k, 2m,n, s–v, 3 s–v, 4a–x, 5a–c, g–x, 6a–h, j, l–s, 7f–u, 9a–p, 10h–u. For gel source data, see Supplementary Fig. 1.
References
Singh, R. & Cuervo, A. M. Autophagy in the cellular energetic balance. Cell Metab. 13, 495–504 (2011).
Donaldson, J. G. & Jackson, C. L. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat. Rev. Mol. Cell Biol. 12, 362–375 (2011).
Yang, J. S. et al. ARFGAP1 promotes the formation of COPI vesicles, suggesting function as a component of the coat. J. Cell Biol. 159, 69–78 (2002).
Lee, S. Y., Yang, J. S., Hong, W., Premont, R. T. & Hsu, V. W. ARFGAP1 plays a central role in coupling COPI cargo sorting with vesicle formation. J. Cell Biol. 168, 281–290 (2005).
Sirover, M. A. On the functional diversity of glyceraldehyde-3-phosphate dehydrogenase: biochemical mechanisms and regulatory control. Biochim. Biophys. Acta 1810, 741–751 (2011).
Yang, J. S. et al. A role for phosphatidic acid in COPI vesicle fission yields insights into Golgi maintenance. Nat. Cell Biol. 10, 1146–1153 (2008).
Yang, J. S. et al. COPI acts in both vesicular and tubular transport. Nat. Cell Biol. 13, 996–1003 (2011).
Park, S. Y., Yang, J. S., Schmider, A. B., Soberman, R. J. & Hsu, V. W. Coordinated regulation of bidirectional COPI transport at the Golgi by CDC42. Nature 521, 529–532 (2015).
Chang, C. et al. AMPK-dependent phosphorylation of GAPDH triggers Sirt1 activation and is necessary for autophagy upon glucose starvation. Mol. Cell 60, 930–940 (2015).
Bai, M. et al. ARFGAP1 promotes AP-2 dependent endocytosis. Nat. Cell Biol. 13, 559–567 (2011).
Li, J. et al. An ACAP1-containing clathrin coat complex for endocytic recycling. J. Cell Biol. 178, 453–464 (2007).
Nie, Z. et al. Specific regulation of the adaptor protein complex AP-3 by the ARF GAP AGAP1. Dev. Cell 5, 513–521 (2003).
Barlowe, C. et al. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77, 895–907 (1994).
Hardie, D. G., Ross, F. A. & Hawley, S. A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 13, 251–262 (2012).
Schaffer, B. E. et al. Identification of AMPK phosphorylation sites reveals a network of proteins involved in cell invasion and facilitates large-scale substrate prediction. Cell Metab. 22, 907–921 (2015).
Kuma, A. et al. The role of autophagy during the early neonatal starvation period. Nature 432, 1032–1036 (2004).
Yang, J. S. et al. A role for BARS at the fission step of COPI vesicle formation from Golgi membrane. EMBO J. 24, 4133–4143 (2005).
Aoe, T. et al. The KDEL receptor, ERD2, regulates intracellular traffic by recruiting a GTPase-activating protein for ARF1. EMBO J. 16, 7305–7316 (1997).
Dai, J. et al. ACAP1 promotes endocytic recycling by recognizing recycling sorting signals. Dev. Cell 7, 771–776 (2004).
Oldham, W. M., Clish, C. B., Yang, Y. & Loscalzo, J. Hypoxia-mediated increases in l-2-hydroxyglutarate coordinate the metabolic response to reductive stress. Cell Metab. 22, 291–303 (2015).
Acknowledgements
This work was supported by grants from the US National Institutes of Health to V.W.H. (GM058615 and GM115683), J.Lo. (HL61795, HL119145, HG007690 and GM107618) and W.M.O. (HL128802), and a grant from the American Heart Association to J.Lo. (D700382).
Reviewer information
Nature thanks D. Hardie and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
J.-S.Y., J.-W.H., S.-Y.P. and J.Li performed colocalization studies. J.-S.Y. performed vesicle reconstitution studies with help from G.V.B. and A.A.M. J.-W.H. performed membrane fractionation studies. J.-S.Y. and J.-W.H. performed ATP and cell death assays. W.M.O. performed metabolic studies with supervision by J.Lo. All authors participated in experimental design and data analysis. V.W.H. supervised the project and wrote the manuscript with input from all other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
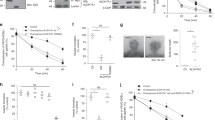
Extended Data Fig. 1 Further characterization of GAPDH inhibition of COPI transport.
a, b, The COPI transport assay was performed on HeLa cells that were treated as indicated. A confocal image from a representative experiment (out of three) is shown. Green, VSVG–KDELR; red, giantin. Scale bar, 10 μm. Line-scan analysis for each image is also shown. c, The COPI transport assay was performed on HeLa cells that were treated as indicated. Quantification of a representative experiment (out of three) is shown, n = 10 fields of cells examined. Data are mean ± s.d. *P = 2.8 × 10−7, **P = 8.2 × 10−7. d, HeLa cells were treated as indicated, and cell death was quantified by flow cytometry. Data are mean ± s.d. from n = 10 independent experiments. P = 0.7314 (NS). e, HeLa cells were treated as indicated, and the COPI transport assay was performed. A confocal image from a representative experiment (out of three) is shown. Green, VSVG–KDELR; red, giantin. Scale bar, 10 μm. Line-scan analysis for each image is also shown. f, HeLa cells were treated as indicated, and whole-cell lysates were immunoblotted for proteins as indicated. Representative blot from three independent experiments. g, GST fusion proteins were incubated with purified GAPDH in a pull-down experiment, followed by immunoblotting as indicated. Representative blot from three independent experiments. h, HeLa cells were transfected with constructs as indicated, followed by immunoprecipitation using the Myc tag and then immunoblotting for GAPDH. Representative blot from two independent experiments. i, GST fusion proteins as indicated were bound to beads and then incubated with purified coatomer in a pull-down experiment, followed by immunoblotting to detect β-COP or Coomassie staining to detect GST fusion proteins. Representative results from three independent experiments. j, The indicated cytoplasmic tails of cargo proteins were fused to GST, bound to beads, and then incubated with ARFGAP1, followed by immunoblotting with antibody against ARFGAP1 or Coomassie staining to detect GST fusion proteins. Representative results from two independent experiments. k, HeLa cells were treated as indicated, and assayed for COPI transport. Quantification of a representative experiment (of three) is shown. Data are mean ± s.d. from n = 10 fields of cells examined. *P = 1.4 × 10−6. l, HeLa cells were transfected with GAPDH constructs as indicated. ARFGAP1 immunoprecipitates were immunoblotted for the different forms of GAPDH as indicated. Representative blot from two experiments. P values determined by two-tailed t-test.
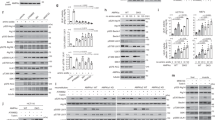
Extended Data Fig. 2 Further characterizing how GAPDH affects other pathways.
a, HeLa cells were treated as indicated, fractionated into cytosol (C) and total membrane (M), and immunoblotted for proteins as indicated. Representative blot from two independent experiments. b–f, HeLa cells were treated as indicated, and examined for the colocalization between GAPDH and different organelle markers. A confocal image from a representative experiment (out of three) is shown. Scale bar, 10 μm (left); quantification of colocalization (right). Data are mean ± s.d. of n = 10 fields of cells examined. Colocalization of GAPDH (red) with giantin (green), *P = 2.9 × 10−4 (b); colocalization of GAPDH (red) with TGN46 (green), *P = 1.5 × 10−4 (c); colocalization of GAPDH (red) with EEA1 (green), *P = 1.9 × 10−3 (d); colocalization of GAPDH (red) with LAMP1 (green), *P = 7.2 × 10−4 (e); colocalization of GAPDH (red) with Sec61p (green), P = 0.8531 (f). g–l, Transport assays were performed in HeLa cells. A confocal image from a representative experiment (out of three) is shown. Scale bar, 10 μm. Line-scan analysis for the representative image is also shown. Transport from the ER to the Golgi (g; green, VSVG; red, giantin), transport from the Golgi to the plasma membrane (h; green, VSVG; red, TGN46), transport from the early endosome to the plasma membrane (i; red, transferrin; green, RAB11), transport from the plasma membrane to the Golgi (j; red, cholera toxin; green, TGN46); transport from the plasma membrane to the lysosome (k; red, EGF; green, LAMP1), transport from the plasma membrane to the early endosome (l; red, dextran; green, EEA1). m, n, Transport assays in HeLa cells; a representative experiment (out of three) is shown, n = 10 fields of cells examined. Data are mean ± s.d. Cholera toxin endocytosis (m), P = 1 (NS1); P = 0.3205 (NS2); interleukin-2 receptor beta subunit (IL2R-β) endocytosis (n), P = 0.063 (NS1), P = 0.9264 (NS2). o–r, HeLa cells were treated as indicated followed by immunofluorescence microscopy using different antibodies against GAPDH (o, q) or imaging of GFP-tagged GAPDH (p, r). Image from a representative experiment of two is shown. Scale bar, 10 μm. P values determined by two-tailed t-test.
Extended Data Fig. 3 Additional characterization of GAPDH and its effects on the transport pathways.
a–d, The distribution of different organelle markers in CHO (a), COS-7 (b), HeLa (c) or MEF (d) cells were assessed by immunofluorescence microscopy. Image from a representative experiment of two experiments is shown. Scale bar, 10 μm. e–h, Comparison of the distribution of two markers against the same intracellular compartment using confocal microscopy. ER markers Sec61p (green) and calnexin (red) (e); Golgi markers giantin (green) and GM130 (red) (f); early endosome markers EEA1 (green) and RAB5 (red) (g); lysosome markers LAMP1 (green) and CD63 (red) (h). Images are representative of two experiments. Scale bar, 10 μm. i–l, Confirming the staining specificity of organelle markers using model cargoes that reside at specific intracellular compartments using confocal microcopy: GFP-tagged VSVG at the ER (green) and Sec61p (red) (i); GFP-tagged VSVG at the Golgi (green) and giantin (red) (j); fluorescently labelled transferrin (green) and EEA (red) (k); fluorescently labelled dextran (green) and LAMP1 (red) (l) Images are representative of two experiments. Scale bar, 10 μm. m, n, The GAP assay was performed using ARF6 and ACAP1 (m) or ARF1 and AGAP1 (n), in the presence of different metabolic enzymes as indicated. One experiment of three independent experiments is shown. o–r, Pull-down studies to detect GAPDH binding directly to: ACAP1 (o), AGAP1 (p), Sec23p (q) or different portions of ARFGAP1 as indicated (r). Representative blot from three experiments. s–v, HeLa (s, u) or BSC-1 (t, v) cells were treated as indicated, followed by transport assay for transferrin endocytosis. Quantification of a representative experiment (out of three) is shown, n = 10 fields of cells examined. Data are mean ± s.d. In s: P = 0.4646 (NS1); P = 0.6973 (NS2). In t: *P = 1.8 × 10−4. In u: P = 0.8073 (NS). In v: *P = 3.6 ×10 −5, **P = 1.7 × 10−6. P values determined by two-tailed t-test.
Extended Data Fig. 4 Different ways of starving cells lead to identical pathways being inhibited and inhibition requires GAPDH.
a–h, Transport assays were performed following incubation of HeLa cells in Hank’s medium. Quantification of one experiment (out of three) is shown. Data are mean ± s.d. of n = 10 fields of cells examined. Transport of VSVG from the ER to the Golgi, P = 0.1944 (NS1), P = 0.05 (NS2) (a); COPI transport of VSVG–KDELR from the Golgi to the ER, *P = 2.4 × 10−6, **P = 4.2 × 10−10 (b); transport of VSVG from the Golgi to the plasma membrane, *P = 1.7 × 10−5, **P = 6.7 × 10−8 (c); transport of transferrin from the early endosome to the plasma membrane, *P = 2.9 × 10−5, **P = 6.9 × 10−9 (d); transport of cholera toxin from the plasma membrane to the Golgi, *P = 2.4 × 10−3, **P = 7.8 × `10−7 (e); transport of EGF from the plasma membrane to the lysosome, *P = 1.9 × 10−6, **P = 1.5 × 10−2 (f); transport of dextran from the plasma membrane to the early endosome, *P = 7.8 × 10−3, **P = 3.4 × × 10−7 (g); transport of EGF from the plasma membrane to the early endosome, P = 0.1485 (NS1), P = 0.6378 (NS2) (h). i–p, HeLa cells were incubated in medium without glucose, and then transport assays were performed. Quantification of one experiment (out of three) is shown. Data are mean ± s.d. from n = 10 fields of cells examined. Transport of VSVG from the ER to the Golgi, P = 0.6921 (NS1), P = 0.5648 (NS2) (i); COPI transport of VSVG–KDELR from the Golgi to the ER, *P = 4.4 × 10−4, **P = 6.5 × 10−7 (j); transport of VSVG from the Golgi to the plasma membrane, *P = 1.6 × 10−2, **P = 6.7 × 10−5 (k); transport of transferrin from the early endosome to the plasma membrane, *P = 1.9 × 10−4, **P = 2.8 × 10−9 (l); transport of cholera toxin from the plasma membrane to the Golgi, *P = 7.4 × 10−4, **P = 4.2 × 10−5 (m); transport of EGF from the plasma membrane to the lysosome, *P = 7.6 × 10−3, **P = 3.2 × 10−9 (n); transport of dextran from the plasma membrane to the early endosome, *P = 2.2 × 10−3, **P = 1.6 × 10−5 (o); transport of EGF from the plasma membrane to the early endosome, P = 0.8648 (NS1), P = 0.8946 (NS2) (p). q–x, HeLa cells were incubated in medium without amino acids, and transport assays were performed. Quantification of a representative experiment (out of three) is shown. Data are mean ± s.d. of n = 10 fields of cells examined. Transport of VSVG from the ER to the Golgi, P = 0.1419 (NS1), P = 0.3379 (NS2) (q); COPI transport of VSVG–KDELR from the Golgi to the ER, *P = 1.9 × 10−5, **P = 1.1 × 10−6 (r); transport of VSVG from the Golgi to the plasma membrane, *P = 1.1 × 10−3, **P = 6.6 × 10−7 (s); transport of transferrin from the early endosome to the plasma membrane, *P = 7.2 × 10−5, **P = 8.4 × 10−7 (t); transport of cholera toxin from the plasma membrane to the Golgi, *P = 1.1 × 10−5, **P = 1.8 × 10−6 (u); transport of EGF from the plasma membrane to the lysosome, *P = 1.1 × 10−2, **P = 9.3 × 10−8 (v); transport of dextran from the plasma membrane to the early endosome, *P = 7.8 × 10−3, **P = 1.2 × 10−8 (w); transport of EGF from the plasma membrane to the early endosome, P = 0.9731 (NS1), P = 0.8159 (NS2) (x). P values determined by two-tailed t-test.
Extended Data Fig. 5 Further characterizing the effects of starvation and GAPDH.
a–c, HeLa cells were starved using Hank’s medium (a), medium lacking glucose (b), or medium lacking amino acids (c), and the total ATP level was measured. Results of one experiment representative of three independent experiments are shown. d, Lactate production, measured in HeLa cells treated as indicated. Results of one experiment representative of three independent experiments are shown. e, Oxygen consumption rate in HeLa cells treated as indicated. Results of one experiment representative of three independent experiments is shown. f, Relative abundance of glycolytic enzymes in HeLa cells. Data are derived from https://pax-db.org; glucose-6-phosphate isomerase (https://pax-db.org/protein/1860535); phosphofructokinase (https://pax-db.org/protein/1852972); fructose-bisphosphate aldolase (https://pax-db.org/protein/1851945); GAPDH (https://pax-db.org/protein/1843185); phosphoglycerate kinase (https://pax-db.org/protein/1848799); phosphoglycerate mutase (https://pax-db.org/protein/1855378); enolase (https://pax-db.org/protein/1850612); pyruvate kinase (https://pax-db.org/protein/1852273). g–n, Transport assays were performed on HEK293 cells that were treated as indicated. Quantification of one representative experiment (out of three) is shown. Data are mean ± s.d. from n = 10 fields of cells examined. Transport of VSVG from the ER to the Golgi, P = 0.6468 (NS1), P = 0.2133 (NS2) (g); COPI transport of VSVG–KDELR from the Golgi to the ER, *P = 4.3 × 10−7, **P = 1.3 × 10−3 (h); transport of VSVG from the Golgi to the plasma membrane, *P = 2.1 × 10−3, **P = 1.9 × 10−5 (i); transport of transferrin from the early endosome to the plasma membrane, *P = 6.3 × 10−5, **P = 4.7 × 10−4 (j); transport of cholera toxin from the plasma membrane to the Golgi, *P = 6.5 × 10−4, **P = 1.5 × 10−2 (k); transport of EGF from the plasma membrane to the lysosome, *P = 7.8 x 10−7, **P = 6.5 × 10−8 (l); transport of dextran from the plasma membrane to the early endosome, *P = 3.6 × 10−6, **P = 3.4 × 10−2 (m); transport of EGF from the plasma membrane to the early endosome, P = 0.2375 (NS1), P = 0.5291 (NS2) (n). o–v, Transport assays were performed on HEK293 cells that were treated as indicated. Quantification of a representative experiment (out of three) is shown. Data are mean ± s.d. from n = 10 fields of cells examined. Transport of VSVG from the ER to the Golgi, P = 0.6649 (NS1), P = 0.84 (NS2) (o); COPI transport of VSVG–KDELR from the Golgi to the ER, *P = 4.6 × 10−3, **P = 2.5 × 10−6 (p); transport of VSVG from the Golgi to the plasma membrane, *P = 7.1 × 10−5, **P = 1.1 × 10−6 (q); transport of transferrin from the early endosome to the plasma membrane, *P = 7.2 × 10−6, **P = 6.5 × 10−9 (r); transport of cholera toxin from the plasma membrane to the Golgi, *P = 5.6 × 10−3, **P = 8.8 × 10−9 (s) transport of EGF from the plasma membrane to the lysosome, *P = 1.1 × 10−2, **P = 2.9 × 10−8 (t); transport of dextran from the plasma membrane to the early endosome, *P = 2.4 × 10−4, **P = 2.4 × 10−6 (u); transport of EGF from the plasma membrane to the early endosome, P = 0.7924 (NS1), P = 0.4675 (NS2) (v). w, x, HEK293 cells were treated as indicated, followed by starvation and quantification of total ATP level (w) or cell death (x). Data are mean ± s.d. of n = 3 experiments. P values determined by two-tailed t-test.
Extended Data Fig. 6 Inhibition of transport pathways by starvation and AMPK.
a–h, HeLa cells were starved using a general starvation medium (lacking glucose and amino acids), and transport assays were performed. Quantification of a representative experiment (out of three) is shown. Data are mean ± s.d. from n = 10 fields of cells examined. Transport of VSVG from the ER to the Golgi, P = 0.3506 (NS1), P = 0.9126 (NS2) (a); COPI transport of VSVG–KDELR from the Golgi to the ER, *P = 8.6 × 10−4, **P = 5.8 × 10−7 (b); transport of VSVG from the Golgi to the plasma membrane, *P = 1.0 × 10−7, **P = 4.4 × 10−9 (c); transport of transferrin from the early endosome to the plasma membrane, *P = 4.2 × 10−5, **P = 4.4 × 10−10 (d); transport of cholera toxin from the plasma membrane to the Golgi, *P = 2.6 × 10−4, **P = 9.5 × 10−7 (e); transport of EGF from the plasma membrane to the lysosome, *P = 2.0 × 10−4, **P = 2.2 × 10−13 (f); transport of dextran from the plasma membrane to the early endosome, *P = 1.4 × 10−4, **P = 1.6 × 10−6 (g); transport of EGF from the plasma membrane to the early endosome, P = 0.1085 (NS1), P = 0.1408 (NS2) (h). i, HeLa cells were treated as indicated and cell lysate was immunoblotted for proteins as indicated. Blot is representative of two independent experiments. j, HeLa cells were starved, and the COPI transport assay was performed. Quantification of a representative experiment (out of three) is shown. Data are mean ± s.d. of n = 10 fields of cells examined. *P = 1.1 × 10−4, **P = 3.5 × 10−6. k, HeLa cells were treated as indicated and the whole-cell lysate was immunoblotted for proteins as indicated. Representative blot of two independent experiments. l–s, HeLa cells were starved, and transport assays were performed. Quantification of a representative experiment (out of three) is shown. Data are mean ± s.d. from n = 10 fields of cells examined. Transport of VSVG from the ER to the Golgi, P = 0.6077 (NS1), P = 0.5535 (NS2) (l); COPI transport of VSVG–KDELR from the Golgi to the ER, *P = 3.5 × 10−6, **P = 3.6 × 10−10 (m); transport of VSVG from the Golgi to the plasma membrane, *P = 4.1 × 10−3, **P = 4.4 × 10−6 (n); transport of transferrin from the early endosome to the plasma membrane, *P = 3.7 × 10−6, **P = 1.1 × 10−9 (o); transport of cholera toxin from the plasma membrane to the Golgi, *P = 7.6 × 10−4, **P = 8.7 × 10−11 (p) transport of EGF from the plasma membrane to the lysosome, *P = 3.1 × 10−8, **P = 1.3 × 10−2 (q) transport of dextran from the plasma membrane to the early endosome, *P = 1.1 × 10−6, **P = 3.7 × 10−10 (r) transport of EGF from the plasma membrane to the early endosome, P = 0.228 (NS1), P = 0.1738 (NS2) (s). P values determined by two-tailed t-test.
Extended Data Fig. 7 Effects of AMPK on GAPDH distribution and transport pathways.
a–e, HeLa cells were treated as indicated, and then examined for colocalization between GAPDH and different organelle markers. A confocal image from a representative experiment (out of three) is shown. Scale bar, 10 μm (left). quantification is also shown (right). Data are mean ± s.d. of n = 10 fields of cells examined. Colocalization of GAPDH (red) with giantin (green), *P = 2.9 × 10−3, P = 0.6397 (NS) (a); colocalization of GAPDH (red) with TGN46 (green), *P = 6.5 × 10−3, P = 0.6413 (NS) (b); colocalization of GAPDH (red) with EEA1 (green), *P = 3.5 × 10−3, P = 0.8793 (NS) (c); colocalization of GAPDH (red) with LAMP1 (green), *P = 9.4 × 10−4, P = 0.551 (NS) (d); colocalization of GAPDH (red) with Sec61p (green), P = 0.5361 (NS1), P = 0.4243 (NS2) (e). f–m, HeLa cells were treated as indicated, and transport assays were performed. Quantification of a representative experiment (out of three) is shown. Data are mean ± s.d. from n = 10 fields of cells examined. Transport of VSVG from the ER to the Golgi, P = 0.5250 (NS1), P = 0.8291 (NS2) (f); COPI transport of VSVG–KDELR from the Golgi to the ER, *P = 4.6 × 10−5, **P = 2.9 × 10−8 (g); transport of VSVG from the Golgi to the plasma membrane, *P = 1.4 × 10−4, **P = 6.9 × 10−10 (h); transport of transferrin from the early endosome to the plasma membrane, *P = 2.0 × 10−7, **P = 1.0 × 10−11 (i) transport of cholera toxin from the plasma membrane to the Golgi, *P = 4.0 × 10−4, **P = 3.9 × 10−8 (j); transport of EGF from the plasma membrane to the lysosome, *P = 7.1 × 10−4, **P = 4.2 × 10−8 (k); transport of dextran from the plasma membrane to the early endosome, *P = 1.4 × 10−7, **P = 1.9 × 10−9 (l); transport of EGF from the plasma membrane to the early endosome, P = 0.6088 (NS1), P = 0.4061 (NS2) (m). n–u, HeLa cells were treated as indicated, and transport assays were performed. Quantification of a representative experiment (out of three) is shown. Data are mean ± s.d. from n = 10 fields of cells examined. Transport of VSVG from the ER to the Golgi, P = 0.5559 (NS1), P = 0.8576 (NS2) (n); COPI transport of VSVG–KDELR from the Golgi to the ER, *P = 4.1 × 10−5, **P = 2.3 × 10−9 (o); transport of VSVG from the Golgi to the plasma membrane, *P = 4.2 × 10−3, **P = 6.3 × 10−5 (p); transport of transferrin from the early endosome to the plasma membrane, *P = 7.2 × 10−4, **P = 3.7 × 10−11 (q) transport of cholera toxin from the plasma membrane to the Golgi, *P = 2.3 × 10−4, **P = 3.0 × 10−8 (r); transport of EGF from the plasma membrane to the lysosome, *P = 2.9 × 10−3, **P = 5.8 × 10−6 (s), transport of dextran from the plasma membrane to the early endosome, *P = 2.9 × 10−2, **P = 1.6 × 10−8 (t) transport of EGF from the plasma membrane to the early endosome, P = 0.6856 (NS1), P = 0.9248 (NS2) (u). P values determined by two-tailed t-test.
Extended Data Fig. 8 Further characterizing how AMPK acts on GAPDH.
a–e, HeLa cells were treated as indicated, and then examined for colocalization between GAPDH and different organelle markers. A confocal image from a representative experiment (out of three) is shown (left) and quantified (right). Scale bar, 10 μm. Data are mean ± s.d. from n = 10 fields of cells examined. Colocalization of GAPDH (red) with giantin (green), *P = 1.5 × 10−4 (a); colocalization of GAPDH (red) with TGN46 (green), *P = 2.2 × 10−4 (b); colocalization of GAPDH (red) with EEA1 (green), *P = 2.1 × 10−3 (c); colocalization of GAPDH (red) with LAMP1 (green), *P = 7.1 × 10−5 (d); colocalization of GAPDH (red) with Sec61p (green), P = 0.9933 (NS) (e). f, Purity of GAPDH forms assessed by Coomassie gel staining. Representative gel from two independent experiments. g, Purity of AMPK complex assessed by Coomassie gel staining. Representative gel from two independent experiments. h–j, AMPK was incubated with wild-type GAPDH (h), GAPDH(S122A) (i), or SAMS, a peptide derived from acetyl-CoA carboxylase (j) in the in vitro kinase assay, and phosphorylation was measured over time. Data are mean ± s.d from a representative experiment of three independent experiments. k, Calculation of the stoichiometry of phosphorylation from the results shown in h–j. l–q, Whole-cell lysates, from HeLa (l–n) or HEK293 (o–q) cells, were treated as indicated and immunoblotted for proteins as indicated. Blots are representative of two independent experiments. P values determined by two-tailed t-test.
Extended Data Fig. 9 Further characterizing how GAPDH mediates the ability of starvation to inhibit the transport pathways.
a–h, HeLa cells were transfected with GAPDH(S122A), followed by starvation. A representative transport assay (of three experiments) is shown. Data are mean ± s.d. from n = 10 fields of cells examined. Transport of VSVG from the ER to the Golgi, P = 0.4144 (NS1), P = 0.7463 (NS2) (a); COPI transport of VSVG–KDELR from the Golgi to the ER, *P = 3.8 × 10−5, **P = 1.9 × 10−9 (b); transport of VSVG from the Golgi to the plasma membrane, *P = 3.6 × 10−3, **P = 1.4 × 10−6 (c); transport of transferrin from the early endosome to the plasma membrane, *P = 1.4 × 10−3, **P = 1.4 × 10−5 (d); transport of cholera toxin from the plasma membrane to the Golgi, *P = 7.9 × 10−5, **P = 1.5 × 10−7 (e); transport of EGF from the plasma membrane to the lysosome, *P = 4.0 × 10−3, **P = 1.3 × 10−9 (f); transport of dextran from the plasma membrane to the early endosome, *P = 3.1 × 10−5, **P = 1.2 × 10−8 (g); transport of EGF from the plasma membrane to the early endosome, P = 0.7549 (NS1), P = 0.2279 (NS2) (h). i–p, HeLa cells were transfected with GAPDH(S122D). A representative transport assay (of three experiments) is shown. Data are mean ± s.d. from n = 10 fields of cells examined. Transport of VSVG from the ER to the Golgi, P = 0.8289 (NS) (i); COPI transport of VSVG–KDELR from the Golgi to the ER, *P = 2.2 × 10−5 (j); transport of VSVG from the Golgi to the plasma membrane, *P = 6.3 × 10−4 (k); transport of transferrin from the early endosome to the plasma membrane, *P = 6.9 × 10−6 (l); transport of cholera toxin from the plasma membrane to the Golgi, *P = 1.3 × 10−4 (m); transport of EGF from the plasma membrane to the lysosome, *P = 1.6 × 10−3 (n); transport of dextran from the plasma membrane to the early endosome, *P = 7.4 × 10−4 (o); transport of EGF from the plasma membrane to the early endosome, P = 0.5473 (NS) (p). P values determined by two-tailed t-test.
Extended Data Fig. 10 Further confirmation that the roles of GAPDH in transport and autophagy are distinct.
a, HeLa cells were transfected with GFP-tagged forms of GAPDH as indicated and examined by immunofluorescence microscopy. A representative image from two independent experiments is shown. Scale bar, 10 μm. b, SIRT1 activity in HeLa cells transfected with GFP-tagged forms of GAPDH as indicated, Data are mean ± s.d. from three independent experiments. c, LC3 puncta formation in HeLa cells transfected with GFP-tagged forms of GAPDH as indicated. Quantification of a representative experiment (of three) is shown. Data are mean ± s.d. from n = 10 fields of cells examined. *P = 2.3 × 10−5, P = 0.5598 (NS). d, LC3 lipidation was assessed by immunoblotting of lysates from HeLa cells transfected with GFP-tagged forms of GAPDH as indicated. A representative blot from two independent experiments is shown. e, LC3 puncta formation in HeLa cells treated as indicated. Quantification of a representative experiment (of three) is shown. Data are mean ± s.d. from n = 10 fields of cells examined. *P = 1.7 × 10−5. f, LC3 lipidation was assessed by immunoblotting of lysates from HeLa cells treated as indicated. A representative blot from two independent experiments is shown. g, p62 level was assessed by immunoblotting of lysates from HeLa cells treated as indicated. A representative blot from two independent experiments is shown. h–o, Transport assays in HeLa cells treated as indicated. Quantification of a representative experiment (of three) is shown. Data are mean ± s.d. from n = 10 fields of cells examined. Transport of VSVG from the ER to the Golgi, P = 0.8155 (NS) (h); COPI transport of VSVG–KDELR from the Golgi to the ER, *P = 1.4 × 10−5 (i); transport of VSVG from the Golgi to the plasma membrane, *P = 5.5 × 10−4 (j); transport of transferrin from the early endosome to the plasma membrane, *P = 3.3 × 10−7 (k); transport of cholera toxin from the plasma membrane to the Golgi, *P = 2.0 × 10−3 (l); transport of EGF from the plasma membrane to the lysosome, *P = 2.4 × 10−6 (m); transport of dextran from the plasma membrane to the early endosome, *P = 3.9 × 10−5 (n); transport of EGF from the plasma membrane to the early endosome, P = 0.7725 (NS) (o). p, q, Total ATP level (p) and cell death (q) in HeLa cells treated as indicated. Data are mean ± s.d. from three independent experiments. r, Total ATP level in HeLa cells treated as indicated. Data are mean ± s.d. from three independent experiments. s, t, COPI transport in HeLa cells treated as indicated. Quantification of a representative experiment (of three) is shown. Data are mean ± s.d. from n = 10 fields of cells examined. *P = 3.5 × 10−5 (s), P = 0.7907 (NS) (t). u, Total ATP level in HeLa cells treated as indicated. Data are mean ± s.d. from three independent experiments. P values determined by two-tailed t-test.
Supplementary information
Supplementary Information
This file contains Supplementary Text, a Supplementary Reference, Supplementary Table 1 and Supplementary Figure 1.
Source data
Rights and permissions
About this article
Cite this article
Yang, JS., Hsu, JW., Park, SY. et al. GAPDH inhibits intracellular pathways during starvation for cellular energy homeostasis. Nature 561, 263–267 (2018). https://doi.org/10.1038/s41586-018-0475-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0475-6
Keywords
This article is cited by
-
Phosphoglycerate kinase 1 acts as a cargo adaptor to promote EGFR transport to the lysosome
Nature Communications (2024)
-
Comprehensive analysis of autophagy-related genes and patterns of immune cell infiltration in valvular atrial fibrillation
BMC Cardiovascular Disorders (2021)
-
Modulation of oxidative phosphorylation augments antineoplastic activity of mitotic aurora kinase inhibition
Cell Death & Disease (2021)
-
The protein kinase Akt acts as a coat adaptor in endocytic recycling
Nature Cell Biology (2020)
-
Dynamic genome evolution and complex virocell metabolism of globally-distributed giant viruses
Nature Communications (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.