Abstract
How our internal state is merged with our visual perception of an impending threat to drive an adaptive behavioural response is not known. Mice respond to visual threats by either freezing or seeking shelter. Here we show that nuclei of the ventral midline thalamus (vMT), the xiphoid nucleus (Xi) and nucleus reuniens (Re), represent crucial hubs in the network controlling behavioural responses to visual threats. The Xi projects to the basolateral amygdala to promote saliency-reducing responses to threats, such as freezing, whereas the Re projects to the medial prefrontal cortex (Re→mPFC) to promote saliency-enhancing, even confrontational responses to threats, such as tail rattling. Activation of the Re→mPFC pathway also increases autonomic arousal in a manner that is rewarding. The vMT is therefore important for biasing how internal states are translated into opposing categories of behavioural responses to perceived threats. These findings may have implications for understanding disorders of arousal and adaptive decision-making, such as phobias, post-traumatic stress and addictions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Yilmaz, M. & Meister, M. Rapid innate defensive responses of mice to looming visual stimuli. Curr. Biol. 23, 2011–2015 (2013).
Fanselow, M. & Lester, L. in Evolution and Learning(eds Bolles R. C. & Beecher M. D.) 185–212 (Lawrence Erlbaum Associates, Hillsdale, 1988).
Eilam, D. Die hard: a blend of freezing and fleeing as a dynamic defense—implications for the control of defensive behavior. Neurosci. Biobehav. Rev. 29, 1181–1191 (2005).
Crawford, M. & Masterson, F. A. Species-specific defense reactions and avoidance learning. An evaluative review. Pavlov. J. Biol. Sci. 17, 204–214 (1982).
Wei, P. et al. Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat. Commun. 6, 6756 (2015).
Scott, J. P. Agonistic behavior of mice and rats: a review. Am. Zool. 6, 683–701 (1966).
Miczek, K. A., Maxson, S. C., Fish, E. W. & Faccidomo, S. Aggressive behavioral phenotypes in mice. Behav. Brain Res. 125, 167–181 (2001).
Unger, E. K. et al. Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep. 10, 453–462 (2015).
Lim, J.-H. A. et al. Neural activity promotes long-distance, target-specific regeneration of adult retinal axons. Nat. Neurosci. 19, 1073–1084 (2016).
Urban, D. J. & Roth, B. L. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu. Rev. Pharmacol. Toxicol. 55, 399–417 (2015).
St John, R. D. Genetic analysis of tail rattling in the mouse. Nature 241, 549–551 (1973).
Curley, J. P., Davidson, S., Bateson, P. & Champagne, F. A. Social enrichment during postnatal development induces transgenerational effects on emotional and reproductive behavior in mice. Front. Behav. Neurosci. 3, 25 (2009).
Beilharz, R. G. & Beilharz, V. C. Observations on fighting behaviour of male mice (Mus musculus L.). Z. Tierpsychol. 39, 126–140 (1975).
Corcoran, K. A. & Quirk, G. J. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J. Neurosci. 27, 840–844 (2007).
Adhikari, A. et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature 527, 179–185 (2015).
Martinez, R. C., Carvalho-Netto, E. F., Ribeiro-Barbosa, E. R., Baldo, M. V. & Canteras, N. S. Amygdalar roles during exposure to a live predator and to a predator-associated context. Neuroscience 172, 314–328 (2011).
Tye, K. M. et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362 (2011).
Weible, A. P., Piscopo, D. M., Rothbart, M. K., Posner, M. I. & Niell, C. M. Rhythmic brain stimulation reduces anxiety-related behavior in a mouse model based on meditation training. Proc. Natl Acad. Sci. USA 114, 2532–2537 (2017).
Junyent, F. & Kremer, E. J. CAV-2—why a canine virus is a neurobiologist’s best friend. Curr. Opin. Pharmacol. 24, 86–93 (2015).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
De Franceschi, G., Vivattanasarn, T., Saleem, A. B. & Solomon, S. G. Vision guides selection of freeze or flight defense strategies in mice. Curr. Biol. 26, 2150–2154 (2016).
Papes, F., Logan, D. W. & Stowers, L. The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell 141, 692–703 (2010).
Root, C. M., Denny, C. A., Hen, R. & Axel, R. The participation of cortical amygdala in innate, odour-driven behaviour. Nature 515, 269–273 (2014).
Lucas, R. J., Douglas, R. H. & Foster, R. G. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat. Neurosci. 4, 621–626 (2001).
McGinley, M. J., David, S. V. & McCormick, D. A. Cortical membrane potential signature of optimal states for sensory signal detection. Neuron 87, 179–192 (2015).
Vinck, M., Batista-Brito, R., Knoblich, U. & Cardin, J. A. Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron 86, 740–754 (2015).
Young, M. J. & Lund, R. D. The anatomical substrates subserving the pupillary light reflex in rats: origin of the consensual pupillary response. Neuroscience 62, 481–496 (1994).
Morel, A., Magnin, M. & Jeanmonod, D. Multiarchitectonic and stereotactic atlas of the human thalamus. J. Comp. Neurol. 387, 588–630 (1997).
Heath, R. G. Electrical self-stimulation of the brain in man. Am. J. Psychiatry 120, 571–577 (1963).
Bishop, M. P., Elder, S. T. & Heath, R. G. Intracranial self-stimulation in man. Science 140, 394–396 (1963).
Krout, K. E., Loewy, A. D., Westby, G. W. & Redgrave, P. Superior colliculus projections to midline and intralaminar thalamic nuclei of the rat. J. Comp. Neurol. 431, 198–216 (2001).
Vertes, R. P. & Martin, G. F. Autoradiographic analysis of ascending projections from the pontine and mesencephalic reticular formation and the median raphe nucleus in the rat. J. Comp. Neurol. 275, 511–541 (1988).
Van der Werf, Y. D., Witter, M. P. & Groenewegen, H. J. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res. Rev. 39, 107–140 (2002).
Stuber, G. D. et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475, 377–380 (2011).
Gross, C. T. & Canteras, N. S. The many paths to fear. Nat. Rev. Neurosci. 13, 651–658 (2012).
Lin, D. et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221-226 (2011).
Acknowledgements
This work was supported by the National Institutes of Health (NIH) National Eye Institute grant R01 EY022157 (A.D.H.), by NIH U01 NS090562 (A.D.H.) and by a National Science Foundation Graduate Research Fellowship (L.D.S.). We thank L. Giacomo and C. Mallory for advice and assistance with electrophysiology.
Reviewer information
Nature thanks D. Lin, H. Shin and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
L.D.S. and A.D.H. conceived the design of the study. L.D.S. and N.I. performed the experiments and analysed the data. L.D.S. and A.D.H. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
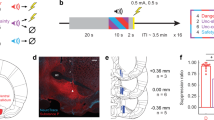
Extended Data Fig. 1 hM3Dq activates vMT, whereas hM4Di inactivates vMT.
Refers to Figs. 1 and 2. a, Timeline of the c-Fos induction protocol. b–d, Recently active c-Fos+ neurons (green) in the vMT of mice that were exposed to the looming stimulus, with XFP (b), hM4Di (c), or hM3Dq (d) injections (red) into the vMT and CNO delivered intraperitoneally. e, After CNO delivery, hM3Dq increased whereas hM4Di decreased the number of c-Fos+ cells in the vMT relative to XFP controls. Scale bars, 100 μm. Data are mean ± s.e.m. *P < 0.05, ***P < 0.001. See Supplementary Table 1 for statistical analysis and sample sizes.
Extended Data Fig. 2 Control groups do not differ in their defensive responses to looming threat.
Refers to Figs. 2–4. a–h, Comparison of different control mice reveals no significant differences across any of the behaviours performed in response to the looming threat: tail rattling (a, e), running (b, f), freezing (c, g), or hiding (d, h). Controls include: mice with no treatments; mice with AAV-XFP and CNO; mice with CAV-Cre, AAV-DIO-XFP and CNO; mice with CAV-Cre and AAV-DIO-hM3Dq but without CNO; and mice with AAV-XFP, optrode implant and sham stimulation. i–l, Comparison of male and female control mice (i, k) and mice with vMT activation (j, l) reveals no significant sex differences across any of the behaviours performed in response to the looming threat. Notably, both male and female mice tail-rattle in response to the looming threat. Data are mean ± s.e.m.; NS, not significant. See Supplementary Table 1 for statistical analysis and sample sizes.
Extended Data Fig. 3 Activating vMT→NAc does not increase tail rattling or saliency-enhancing behaviours.
Refers to Fig. 3. a–d, Mice were injected with AAV-GFP in the vMT (n = 17 mice; a, b) and axons were observed in the NAc (c, d). Representative image of GFP+ neurons in the VMT (b) and GFP+ axons in the NAc (d). e, To activate vMT neurons that project to the NAc, CAV-Cre was injected into the NAc and Cre-dependent hM3Dq was injected into the vMT. f, Representative image of hM3Dq+ neurons in the vMT that project to the NAc. g, Activating the vMT→NAc pathway did not significantly change the behavioural responses to looming as compared to controls. Scale bars, 100 μm. Data are mean ± s.e.m.; NS, not significant. See Supplementary Table 1 for statistical analysis and sample sizes.
Extended Data Fig. 4 Viral targeting and number of cells infected in the vMT.
Refers to Figs. 3 and 4. a, To activate vMT neurons that project to the mPFC or the BLA, CAV-Cre was injected into the mPFC or the BLA and Cre-dependent hM3D was injected into the vMT. b, The average number of infected hM3d–mCherry+ vMT cells did not differ between the vMT→BLA and the vMT→mPFC pathway activation groups. c, e, Locations of injections to activate the vMT→mPFC pathway. d, Relative expression of hM3D in vMT→mPFC cells does not scale with tail-rattling behaviour. f, g, Representative images of hM3dq–mCherry/Cre+ neurons (red) in the vMT that project to the mPFC. h, Mice were injected with AAV-ChR2 in the vMT to activate the vMT. i, j, Representative images of ChR2–eYFP+ neurons (green) in the vMT and the fibre tracts composed of vMT axons that project to the mPFC. Data are mean ± s.e.m.; NS, not significant. See Supplementary Table 1 for statistical analysis and sample sizes.
Extended Data Fig. 5 vMT activation results in saliency-enhancing behaviours performed in the open arena.
Refers to Figs. 3 and 4. a, Percentage of mice tail-rattling in response to looming after vMT→PFC or vMT→BLA activation. All of the mice with vMT→mPFC optogenetic stimulation displayed tail-rattling behaviour. b, c, Percentage of tail-rattling (b) or running (c) events performed in the open arena as opposed to in the shelter. Mice with vMT→ mPFC optogenetic stimulation perform most tail-rattling and running events in the open. d, Optogenetic activation of the vMT results in most mice tail-rattling. e, Mice with vMT optogenetic stimulation perform most tail-rattling events in the open arena as opposed to in the shelter. f, Mice with vMT optogenetic stimulation perform most running events within the open arena as opposed to towards the shelter. Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001; NS, not significant. See Supplementary Table 1 for statistical analysis and sample sizes.
Extended Data Fig. 6 vMT activation does not change locomotion, aggression or anxiety, but does result in less freezing in response to a predator odour.
Refers to Figs. 4 and 5. a, Mice were injected with AAV-ChR2 in the vMT in order to activate the vMT during presentation of the predator-odour threat. b, Activating the vMT (n = 9 mice) decreased freezing as compared to controls with sham stimulation (n = 7 mice). c, Activating the vMT decreased avoidance as compared to controls with sham stimulation (avoidance index = (P − 50)/50, where P is the percentage of time the mouse spent on the side of the arena away from the odour). d, The visual cliff test. e, Activating the vMT (hM3D + CNO, n = 15 mice) or inactivating the vMT (hM4D + CNO, n = 9) during the visual cliff test did not change the number of times in which the mouse chose the shallow side as compared to control mice (n = 9 mice, P < 0.05). f, The RTPP test. g, Activating the vMT (ChR2, n = 7 mice) did not change the relative activity (distance covered) in the RTPP test as compared to control mice (n = 6 mice). h, Mice were tested on the resident-intruder test for aggressive behaviours. i, Activating the vMT (ChR2, n = 7 mice) did not change the average number of tail-rattling events in the resident-intruder test as compared to control mice (n = 14 mice). j, k, Activating the vMT (ChR2, n = 7 mice) did not change the percentage of mice attacking (j) or the latency to attack (k) in the resident-intruder test as compared to control mice (n = 14 mice). l, Mice were subjected to an open field test to analyse anxiety-related behaviour; representative tracing of a control mouse (AAV-GFP, left) and a mouse with vMT activation (AAV-ChR2, right) in the open field test. m, Activating the vMT (ChR2, n = 10 mice) did not change the percentage of time in the centre of the open field as compared to control mice (n = 10 mice). n, Activating the vMT (ChR2, n = 10 mice) did not change the relative activity (distance covered) in the open field test as compared to control mice (n = 10 mice). Data are mean ± s.e.m. *P < 0.05; NS, not significant. See Supplementary Table 1 for statistical analysis and sample sizes.
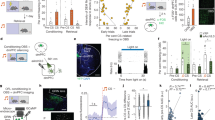
Extended Data Fig. 7 vMT activation results in increased arousal.
Refers to Fig. 5. a, Schematic of the experimental set-up to analyse light-driven pupillary responses in mice. Treating control mice with CNO did not change pupil size across all light levels. b, Chronic vMT activation by chemicogenetic methods (+CNO) significantly increases pupil size across all light levels as compared to the same mice without activation (−CNO). c, Chronic vMT inactivation (+CNO) did not the change pupil size across all light levels as compared to the same mice without activation (−CNO). d, Schematic of experimental set-up to analyse arousal-driven pupillary responses in mice with vMT activation, mice with vMT inactivation and control mice. The pupil was measured in constant light (100 lx) conditions in mice with and without CNO. e, After CNO delivery, mice with hM3Dq had a significant increase in relative pupil size. Mice with XFP and hM4Di did not have a significant change in pupil size after administration of CNO. f, In constant dark conditions, vMT activation significantly increased pupil size, whereas vMT inactivation did not change pupil size as compared to control mice with CNO. g, In constant dark conditions, optogentic activation of the vMT significantly increased pupil size (n = 11 mice) compared to control mice (n = 12 mice). Data are mean ± s.e.m. *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant. See Supplementary Table 1 for statistical analysis and sample sizes.
Extended Data Fig. 8 Looming stimuli induce vMT activation in naive, but not experienced mice habituated to looms.
Refers to Fig. 6. a, Schematic of the experimental protocol. b, c, Quantification of c-Fos+ cells in the vMT of naive or pre-exposed mice that experienced looming from above revealed a significant decrease in c-Fos+ cells in the vMT (b) and Xi (c) after looming from above in pre-exposed mice that are habituated to the looms (n = 6 mice) as compared to naive mice (n = 7 mice). Data are mean ± s.e.m. **P < 0.01; ***P < 0.001. Supplementary Table 1 for statistical analysis and sample sizes.
Extended Data Fig. 9 Tail rattling and running scales with arousal.
Refers to Figs. 4 and 5. a, b, Mice in which ChR2 had been injected into the vMT were divided into three groups based on the extent to which vMT activation increased arousal responses: low, moderate or high (n = 7, n = 9 and n = 10 mice, respectively). Mice with high arousal had significantly more tail-rattling (a) and running (b) events in response to looming, as compared to mice with low arousal (P < 0.001 and P < 0.001). c, d, Mice with high arousal spent similar amounts of time freezing (c) and hiding (d) in response to looming, as compared to mice with low arousal. e, f, 100% of mice with high arousal tail-rattled (e) and ran (f) in response to looming, whereas only 33% and 20% of mice with low arousal tail-rattled and ran, respectively. g, h, Similar numbers of mice with high arousal froze (g) and hid (h) in response to looming as compared to mice with low arousal. Data are mean ± s.e.m. *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant. See Supplementary Table 1 for statistical analysis and sample sizes.
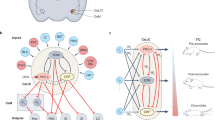
Extended Data Fig. 10 Hindbrain inputs to the vMT.
a, Schematic illustration of ΔG-rabies-XFP injection into the vMT to map vMT inputs. b, Quantification of the relative density of projection neurons to the vMT (n = 12 mice). c–e, Representative images showing the expression of ΔG-rabies-XFP in transynaptically labelled cells in the superior colliculus (c), dorsal raphe (d), periaqueductal grey (d), and median raphe (e). DRN, dorsal raphe; MRN, median raphe; PAG, periaqueductal grey; PRN, pontine reticular nucleus; SCm, superior colliculus, motor; SCs, superior colliculus, sensory. Scale bars, 100 μm.
Supplementary information
Supplemental Table 1
This table contains the statistical analysis
Supplemental Video 1
Looming stimuli cause freeze behavior (refers to Figure 1) Control mice freeze in response to overhead looming stimuli. Refer to figure 1 for quantification
Supplemental Video 2
Looming stimuli cause escape behavior (refers to Figure 2) Control mice also run to the provided shelter in order to hide in response to overhead looming stimuli. Refer to figure 1 for quantification
Supplemental Video 3
vMT inactivation promotes saliency-reducing responses to looming stimuli (refers to Figure 3) Mice with vMT inactivation freeze in response to overhead looming stimuli. Refer to figure 2 for quantification
Supplemental Video 4
vMT activation promotes saliency-enhancing responses to looming stimuli (refers to Figure 4) Mice with vMT activation tail rattle within the open arena in response to overhead looming stimuli. Refer to figure 2 for quantification
Supplemental Video 5
Optogenetic activation of the vMT promotes saliency-enhancing responses to looming stimuli (refers to Figure 5) Mice with optogenetic vMT activation tail rattle and run within the open arena in response to overhead looming stimuli. Refer to figure 4 for quantification
Rights and permissions
About this article
Cite this article
Salay, L.D., Ishiko, N. & Huberman, A.D. A midline thalamic circuit determines reactions to visual threat. Nature 557, 183–189 (2018). https://doi.org/10.1038/s41586-018-0078-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0078-2
This article is cited by
-
Neural basis of why mammals eat more in the cold
Nature (2023)
-
Experimenter familiarization is a crucial prerequisite for assessing behavioral outcomes and reduces stress in mice not only under chronic pain conditions
Scientific Reports (2023)
-
Neurofunctional underpinnings of individual differences in visual episodic memory performance
Nature Communications (2023)
-
Xiphoid nucleus of the midline thalamus controls cold-induced food seeking
Nature (2023)
-
A melanopsin ganglion cell subtype forms a dorsal retinal mosaic projecting to the supraoptic nucleus
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.