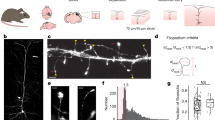
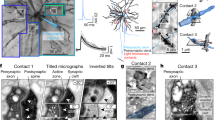
Abstract
Research on neuronal connectivity in the cerebral cortex has focused on the existence and strength of synapses between neurons, and their location on the cell bodies and dendrites of postsynaptic neurons. The synaptic architecture of individual presynaptic axonal trees, however, remains largely unknown. Here we used dense reconstructions from three-dimensional electron microscopy in rats to study the synaptic organization of local presynaptic axons in layer 2 of the medial entorhinal cortex, the site of grid-like spatial representations. We observe path-length-dependent axonal synapse sorting, such that axons of excitatory neurons sequentially target inhibitory neurons followed by excitatory neurons. Connectivity analysis revealed a cellular feedforward inhibition circuit involving wide, myelinated inhibitory axons and dendritic synapse clustering. Simulations show that this high-precision circuit can control the propagation of synchronized activity in the medial entorhinal cortex, which is known for temporally precise discharges.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kasthuri, N. et al. Saturated reconstruction of a volume of neocortex. Cell 162, 648–661 (2015)
Ahmed, B., Anderson, J. C., Martin, K. A. & Nelson, J. C. Map of the synapses onto layer 4 basket cells of the primary visual cortex of the cat. J. Comp. Neurol. 380, 230–242 (1997)
Holtmaat, A., Wilbrecht, L., Knott, G. W., Welker, E. & Svoboda, K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature 441, 979–983 (2006)
Knott, G. W., Holtmaat, A., Wilbrecht, L., Welker, E. & Svoboda, K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat. Neurosci. 9, 1117–1124 (2006)
Mishchenko, Y. et al. Ultrastructural analysis of hippocampal neuropil from the connectomics perspective. Neuron 67, 1009–1020 (2010)
Koganezawa, N., Gisetstad, R., Husby, E., Doan, T. P. & Witter, M. P. Excitatory postrhinal projections to principal cells in the medial entorhinal cortex. J. Neurosci. 35, 15860–15874 (2015)
van Haeften, T., Baks-te-Bulte, L., Goede, P. H., Wouterlood, F. G. & Witter, M. P. Morphological and numerical analysis of synaptic interactions between neurons in deep and superficial layers of the entorhinal cortex of the rat. Hippocampus 13, 943–952 (2003)
Markram, H., Lübke, J., Frotscher, M., Roth, A. & Sakmann, B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J. Physiol. (Lond.) 500, 409–440 (1997)
Markram, H., Lübke, J., Frotscher, M. & Sakmann, B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275, 213–215 (1997)
Feldmeyer, D., Egger, V., Lubke, J. & Sakmann, B. Reliable synaptic connections between pairs of excitatory layer 4 neurones within a single ‘barrel’ of developing rat somatosensory cortex. J. Physiol. (Lond.) 521, 169–190 (1999)
Feldmeyer, D., Lübke, J., Silver, R. A. & Sakmann, B. Synaptic connections between layer 4 spiny neurone-layer 2/3 pyramidal cell pairs in juvenile rat barrel cortex: physiology and anatomy of interlaminar signalling within a cortical column. J. Physiol. (Lond.) 538, 803–822 (2002)
Helmstaedter, M., Staiger, J. F., Sakmann, B. & Feldmeyer, D. Efficient recruitment of layer 2/3 interneurons by layer 4 input in single columns of rat somatosensory cortex. J. Neurosci. 28, 8273–8284 (2008)
Jiang, X. et al. Principles of connectivity among morphologically defined cell types in adult neocortex. Science 350, aac9462 (2015)
Couey, J. J. et al. Recurrent inhibitory circuitry as a mechanism for grid formation. Nat. Neurosci. 16, 318–324 (2013)
Fuchs, E. C. et al. Local and distant input controlling excitation in layer II of the medial entorhinal cortex. Neuron 89, 194–208 (2016)
Briggman, K. L., Helmstaedter, M. & Denk, W. Wiring specificity in the direction-selectivity circuit of the retina. Nature 471, 183–188 (2011)
Helmstaedter, M. et al. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 500, 168–174 (2013)
Wanner, A. A., Genoud, C., Masudi, T., Siksou, L. & Friedrich, R. W. Dense EM-based reconstruction of the interglomerular projectome in the zebrafish olfactory bulb. Nat. Neurosci. 19, 816–825 (2016)
Bock, D. D. et al. Network anatomy and in vivo physiology of visual cortical neurons. Nature 471, 177–182 (2011)
Lee, W. C. et al. Anatomy and function of an excitatory network in the visual cortex. Nature 532, 370–374 (2016)
Helmstaedter, M. Cellular-resolution connectomics: challenges of dense neural circuit reconstruction. Nat. Methods 10, 501–507 (2013)
Denk, W. & Horstmann, H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2, e329 (2004)
Helmstaedter, M., Briggman, K. L. & Denk, W. High-accuracy neurite reconstruction for high-throughput neuroanatomy. Nat. Neurosci. 14, 1081–1088 (2011)
Hua, Y., Laserstein, P. & Helmstaedter, M. Large-volume en-bloc staining for electron microscopy-based connectomics. Nat. Commun. 6, 7923 (2015)
Ray, S. et al. Grid-layout and theta-modulation of layer 2 pyramidal neurons in medial entorhinal cortex. Science 343, 891–896 (2014)
Kitamura, T. et al. Island cells control temporal association memory. Science 343, 896–901 (2014)
Boergens, K. M. et al. webKnossos: efficient online 3D data annotation for connectomics. Nat. Methods 14, 691–694 (2017)
de Vivo, L. et al. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science 355, 507–510 (2017)
Harris, K. M. & Stevens, J. K. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J. Neurosci. 9, 2982–2997 (1989)
Arellano, J. I., Benavides-Piccione, R., Defelipe, J. & Yuste, R. Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies. Front. Neurosci. 1, 131–143 (2007)
Bopp, R., Holler-Rickauer, S., Martin, K. A. & Schuhknecht, G. F. An ultrastructural study of the thalamic input to layer 4 of primary motor and primary somatosensory cortex in the mouse. J. Neurosci. 37, 2435–2448 (2017)
Staffler, B. et al. SynEM, automated synapse detection for connectomics. eLife 6, e26414 (2017)
Beed, P. et al. Analysis of excitatory microcircuitry in the medial entorhinal cortex reveals cell-type-specific differences. Neuron 68, 1059–1066 (2010)
Bruno, R. M. & Simons, D. J. Feedforward mechanisms of excitatory and inhibitory cortical receptive fields. J. Neurosci. 22, 10966–10975 (2002)
Cruikshank, S. J., Lewis, T. J. & Connors, B. W. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat. Neurosci. 10, 462–468 (2007)
Kanichay, R. T. & Silver, R. A. Synaptic and cellular properties of the feedforward inhibitory circuit within the input layer of the cerebellar cortex. J. Neurosci. 28, 8955–8967 (2008)
Eccles, J., Llinas, R. & Sasaki, K. Golgi cell inhibition in the cerebellar cortex. Nature 204, 1265–1266 (1964)
Buzsáki, G. Feed-forward inhibition in the hippocampal formation. Prog. Neurobiol. 22, 131–153 (1984)
Alger, B. E. & Nicoll, R. A. Feed-forward dendritic inhibition in rat hippocampal pyramidal cells studied in vitro. J. Physiol. (Lond.) 328, 105–123 (1982)
Pouille, F. & Scanziani, M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293, 1159–1163 (2001)
Duguid, I. et al. Control of cerebellar granule cell output by sensory-evoked Golgi cell inhibition. Proc. Natl Acad. Sci. USA 112, 13099–13104 (2015)
Micheva, K. D. et al. A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons. eLife 5, e15784 (2016)
Hoffmann, J. H. et al. Synaptic conductance estimates of the connection between local inhibitor interneurons and pyramidal neurons in layer 2/3 of a cortical column. Cereb. Cortex 25, 4415–4429 (2015)
Koelbl, C., Helmstaedter, M., Lübke, J. & Feldmeyer, D. A barrel-related interneuron in layer 4 of rat somatosensory cortex with a high intrabarrel connectivity. Cereb. Cortex 25, 713–725 (2015)
Takács, V. T., Klausberger, T., Somogyi, P., Freund, T. F. & Gulyás, A. I. Extrinsic and local glutamatergic inputs of the rat hippocampal CA1 area differentially innervate pyramidal cells and interneurons. Hippocampus 22, 1379–1391 (2012)
Kress, G. J., Dowling, M. J., Meeks, J. P. & Mennerick, S. High threshold, proximal initiation, and slow conduction velocity of action potentials in dentate granule neuron mossy fibers. J. Neurophysiol. 100, 281–291 (2008)
Schmidt-Hieber, C., Jonas, P. & Bischofberger, J. Action potential initiation and propagation in hippocampal mossy fibre axons. J. Physiol. (Lond.) 586, 1849–1857 (2008)
Bruno, R. M. Synchrony in sensation. Curr. Opin. Neurobiol. 21, 701–708 (2011)
Alonso, A. & Klink, R. Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer II. J. Neurophysiol. 70, 128–143 (1993)
Alonso, A. & Llinás, R. R. Subthreshold Na+-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer II. Nature 342, 175–177 (1989)
Preibisch, S., Saalfeld, S. & Tomancak, P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 25, 1463–1465 (2009)
Burgalossi, A. et al. Microcircuits of functionally identified neurons in the rat medial entorhinal cortex. Neuron 70, 773–786 (2011)
Helmstaedter, M., Sakmann, B. & Feldmeyer, D. The relation between dendritic geometry, electrical excitability, and axonal projections of L2/3 interneurons in rat barrel cortex. Cereb. Cortex 19, 938–950 (2009)
Gupta, A., Wang, Y. & Markram, H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science 287, 273–278 (2000)
Markram, H. et al. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 5, 793–807 (2004)
Acknowledgements
We thank G. Laurent for discussions, A. Borst, A. Motta, R. Rao and A. T. Schaefer for comments on the manuscript, W. Denk for providing the SBEM microtome, Y. Hua for advice on staining protocols, U. Schneeweis for technical help with calbindin staining, M. Berning, E. Klinger and B. Staffler for contributions to data alignment, H. Wissler and D. Rustemovic for tracer management, F. Haake and R. Gebauer for tracing review, E. Eulig, R. Hesse, C. Schramm and M. Zecevic for data curation and J. Abramovich, N. Aydin, N. Berghaus, M. Dell, T. Engelmann, K. Friedl, M. Groothuis, J. Hartel, M.-L. Harwardt, J. Heller, M. Karabel, D. Kurt, E. Laubender, F. Lautenschlager, K. Lust, J. Lösch, L. Matzner, J.-P. Poths, M. Präve, S. Roth, F. Sahin, D. J. Scheliu, N. Schmidt, J. Schmidt-Engler, L. Schütz, S. Sternkopf, A. Strubel, H. Suliman and P. Werner for neuron tracing.
Author information
Authors and Affiliations
Contributions
M.H. and M.B. conceived and supervised the study; H.S. carried out experiments with contributions from A.G.; H.S. analysed the data with contributions from M.H.; K.M.B. contributed to experimental methods; J.S. and M.H. performed circuit modelling; M.H., H.S. and M.B. wrote the paper with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks A. Konnerth, M. Witter and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 P90 dataset, continuous SBEM imaging and calbindin (CB) immunohistochemistry.
a, Dimensions of the P90 SBEM dataset (pia, top). Dashed bounding box: initial 101 μm in z after which the analysis was started. b–f, Continuous SBEM imaging. b, Sketch of microtome with piezo actor installed in-line with the geared motor (see Methods). c–e, Sketch of the stage movement and imaging setup in continuous SBEM imaging. f, Piezo actor command voltages during one motortile acquisition. See Methods for details. g, h, Confirmation of sample location in the dorsal MEC. Post hoc CB+ staining of the remaining tissue after electron microscopy (EM) sample extraction for the P25 dataset (g) and P90 dataset (h). CB+ patches are visible close to the pia. HC, hippocampus.
Extended Data Figure 2 Gallery of electron-microscopy-based reconstructions from P25 dataset.
a, Pyramidal cells (top) and stellate cells (bottom) for which expert consensus was reached about cell-type classification. Note apical dendrites (magenta arrows) and denser plexus of basal dendrites for pyramidal cells, and lack of a clear apical dendrite for stellate cells. b, Same for locally reconstructed neurons. Scale bar, 100 μm.
Extended Data Figure 3 PLASS with regards to cell types and synapse positions in relation to patches and the cortical axis of the MEC.
See Fig. 2. a, Position of output synapses along presynaptic ExN axons analysed separately for consensus pyramidal cells (top; n = 6 axons, n = 19 (synapses onto excitatory cells) versus n = 19 (inhibitory targets), 222 ± 32 μm versus 173 ± 38 μm, mean ± s.d., t-test, Wilcoxon rank-sum test and randomization test, P < 10−3), stellate cells (middle; n = 4 axons, n = 36 (synapses onto excitatory cells) versus n = 51 (inhibitory targets), 303 ± 58 μm versus 249 ± 53 μm, mean ± s.d., t-test, Wilcoxon rank-sum test, P < 10−4; randomization test, P < 10−3) and intermediate types (bottom; n = 5 axons, n = 81 (synapses onto excitatory cells) versus n = 70 (inhibitory targets), 257 ± 68 μm versus 202 ± 75 μm, mean ± s.d., t-test, Wilcoxon rank-sum test, P < 10−5; randomization test, P < 10−3). Note that all ExNs exhibit PLASS. b, Reconstruction of all myelinated axons (black) in L1 and upper L2, projected in the tangential plane. Yellow, circular areas of low myelin density previously identified with the patches of CB+ neurons in the MEC25. c, d, Distribution of distance of synapses to each of the three patch centres (c) and to the nearest patch centre (d). Note that no distance bias for interneuron-targeting (black) versus ExN-targeting (magenta) synapses can be seen. e, Output synapses along the radial cortex axis (cortical depth, histogram on the left) and in paracoronal plane of the MEC (right, plane of imaging; see Fig. 1a). The synapse distribution along the cortical depth shows a bias of inhibitory targets towards deeper L2 (n = 15 axons, n = 136 (synapses onto excitatory cells) versus n = 140 (inhibitory targets), 223 ± 47 μm versus 189 ± 45 μm, mean ± s.d., t-test, P < 10−8, reported as the relative position to the bottom of the dataset). Green, location of seven cubes with a size of 10 × 10 × 10 μm3 each in which dendrites were densely reconstructed. Green circles, dense reconstructions shown in f. f, Left, fraction of ExN output synapses made onto dendritic shafts (black) versus spines (magenta) over cortical depth. The fractional path length of smooth dendrites sampled at seven different cortical locations is also shown (green, examples on the right). Note that while the fraction of smooth dendrites is about twofold higher in lower L2 than in upper L2, its gradient cannot account for the about fourfold bias of output synapses onto interneurons in lower L2 (black versus green lines). Green dashed line, linear fit to the interneuron dendrite fraction (n = 7, see Methods). Right, skeleton reconstructions of all dendrites within a 10 × 10 × 10 μm3 cube sampled at about 150 μm (top) and about 60 μm (bottom) from the L1/2 border. Interneuron dendrites, black; ExN dendrites, magenta. Scale bars, 100 μm (b) and 50 μm (e, f).
Extended Data Figure 4 Functional comparison of population and cellular feedforward inhibition (simulations).
See also Fig. 3. a, Sketch of an example circuit converging onto a postsynaptic ExN (magenta square) in population feedforward inhibition (pFFI) comprising a pool of presynaptic ExNs (light magenta) that target the postsynaptic ExN, in parallel to a pool of presynaptic ExNs (grey) from the same presynaptic population that target a pool of interneurons, which in turn converge onto the postsynaptic ExN. Note that in the case of pure pFFI, the two sets of presynaptic neurons are disjunct (light magenta versus grey). b, Simulated spike histograms for the presynaptic populations (left), the resulting simulated spike distribution in the interneuron population (single neurons, top; summed histogram over 100 trials, bottom) and the resulting simulated spike distribution in the target ExN (top right, four example cells). Note that the statistics of spiking in presynaptic population are indistinguishable between both presynaptic populations (left). c, Sketch of an example circuit configured as cFFI. Note that presynaptic neurons that innervate the postsynaptic ExN are the same ones that innervate the pool of interneurons (as shown in Fig. 3). d, Example simulated spike distribution (top, four modelled neurons, six repetitions shown) and average spike histogram per stimulus (bottom) in cFFI configuration. The spike distribution of the presynaptic ExN population is as in b, grey panel; and therefore the interneuron spike distribution is as in b, black panel. Note the more narrow distribution and lower spike rate compared to pFFI (b). e, Average simulated spike histogram of four cells (aligned to median per cell), 2,000 trials each, for the conditions: no inhibition (blue), pFFI (black), cFFI (red). Arrows indicate width between 25th and 75th percentile. f, g, Quantification of cFFI versus pFFI effects on the width of the postsynaptic timing distribution of action potentials (f) and the number of action potentials (g). Note that cFFI further suppresses the rate of action potentials compared to pFFI (g; by twofold, 0.16 ± 0.02 (cFFI) versus 0.37 ± 0.04 (pFFI) action potentials per cell per trial, mean ± s.d., t-test, ***P < 10−22) and narrows the timing of action potentials (f; by 1.8 ms, width of spike time histogram 7.8 ± 1.2 ms (cFFI) from 9.7 ± 1.1 ms under pFFI, mean ± s.d., n = 2,000 trials per cell, t-test, ***P < 10−4). h, i, Stability of the effect of cFFI on spike timing and spike rate under variation of excitatory and inhibitory circuit convergence. h, The effect of cFFI on spike timing measured as the decrease in spike histogram width (see e, f); relative reduction in 75th-to-25th percentile width is reported for cFFI versus pFFI (P < 0.01 for NExN = 50–80 and NIN = 5–7, t-test over 1,000 trials per postsynaptic cell). i, Relative reduction in spike rate in cFFI compared to pFFI. Note that the spike rate reduction is most substantial (more than twofold) for presynaptic pool sizes of NExN = 60–80 and NIN = 7–10 (P < 10−5 for NExN = 30–80 and NIN = 7–10; P < 0.05 for NIN = 5; t-test over 1,000 trials per postsynaptic cell). j, The effect of presynaptic spike rate on cFFI. Note that for a range of 50–90 Hz presynaptic activity, both the time histogram width and the rate of action potentials are significantly reduced compared to pFFI (**P < 0.01 for histogram width of action potentials; P < 10−6 for the spike rate of action potentials; one-sided t-test against 1). Data are mean ± s.d. (f, g).
Extended Data Figure 5 Axonal architecture of interneurons.
See Fig. 5. a, Morphologies of three interneurons (see Fig. 5b) involved in cFFI circuits. From left to right: 4.5, 2.3 and 3.6 mm reconstructed axonal path length (red), respectively. Scale bar,100 μm. b, Development of axon diameters along the trajectory between soma and distal synapses for one interneuron (n = 7 synapses, grey traces) and for three ExNs (n = 12 synapses, magenta traces) from the P90 dataset. Mean and s.e.m. at intervals of 25 μm distance are shown (based on linear interpolation between diameter measurement locations, see Methods). Note the about 2.7-fold larger diameter of interneuron axons between about 83 and 188 μm path-length distance (indicated by black arrows, n = 12 (excitatory) versus n = 7 (inhibitory) paths to synapse, 0.4 ± 0.1 μm versus 1.09 ± 0.46 μm, mean ± s.d., t-test, P < 10−3, Wilcoxon rank-sum test, P < 10−4).
Extended Data Figure 6 Numerical simulations of the PLASS–cFFI circuit motif.
See Fig. 6. a, The effect of PLASS on suppression of synchronized activity, for a synchronization interval Δtsync of 10 ms (compare with Fig. 6g, i). b, c, The effect of additional background activity on PLASS-based suppression of synchronized activity propagation. Example shows 20 Hz background activity, under which PLASS-based suppression is still effective for synchronization intervals 3 and 10 ms. d–h, The effect of an additional postsynaptic pre-depolarization on recovery from PLASS-induced supression. d, Emulated current injection in the postsynaptic neuron for a PLASS circuit with 0.7 ms inhibitory delay and 1 ms PLASS delay. e, Presynaptic synchronized activity and 8-ms-long rectangular pre-depolarizations in the postsynaptic neuron. f, Simulated membrane potential transients in the postsynaptic excitatory neuron. Note spike suppression by PLASS (magenta, no additional stimulation), that can be gradually recovered from by current injections of increasing amplitude Istim. g, h, Titration of PLASS recovery over stimulus strength and effective pre-depolarization before the synchronized input activity. A pre-depolarization of 10–20 mV is sufficient for the recovery from PLASS; this could be achieved by underlying membrane potential modulation or an additional gating input to the postsynaptic excitatory neuron, for example at its apical dendrites in L1.
Supplementary information
Supplementary Data 1
Neuronal adjacency matrix (local connectome) reporting the number of synapses between all reconstructed pre- and postsynaptic processes in P25 and P90 datasets (xls-sheet). See Methods and matlab code in Supplementary Data 2 for analysis of this data. (XLSX 47 kb)
Supplementary Data 2
This file contains the matlab code for evaluation of cFFI circuit motifs based on connectivity data reported in Supplementary Data 1 (see Methods for details). (TXT 2 kb)
Rights and permissions
About this article
Cite this article
Schmidt, H., Gour, A., Straehle, J. et al. Axonal synapse sorting in medial entorhinal cortex. Nature 549, 469–475 (2017). https://doi.org/10.1038/nature24005
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature24005
This article is cited by
-
RoboEM: automated 3D flight tracing for synaptic-resolution connectomics
Nature Methods (2024)
-
Positive and biphasic extracellular waveforms correspond to return currents and axonal spikes
Communications Biology (2023)
-
Multi-layered maps of neuropil with segmentation-guided contrastive learning
Nature Methods (2023)
-
Brain compartmentalization based on transcriptome analyses and its gene expression in Octopus minor
Brain Structure and Function (2023)
-
Functional and multiscale 3D structural investigation of brain tissue through correlative in vivo physiology, synchrotron microtomography and volume electron microscopy
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.