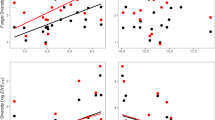
Abstract
Research on biodiversity and ecosystem functioning has demonstrated links between plant diversity and ecosystem functions such as productivity1,2. At other trophic levels, the plant microbiome has been shown to influence host plant fitness and function3,4, and host-associated microbes have been proposed to influence ecosystem function through their role in defining the extended phenotype of host organisms5,6 However, the importance of the plant microbiome for ecosystem function has not been quantified in the context of the known importance of plant diversity and traits. Here, using a tree biodiversity–ecosystem functioning experiment, we provide strong support for the hypothesis that leaf bacterial diversity is positively linked to ecosystem productivity, even after accounting for the role of plant diversity. Our results also show that host species identity, functional identity and functional diversity are the main determinants of leaf bacterial community structure and diversity. Our study provides evidence of a positive correlation between plant-associated microbial diversity and terrestrial ecosystem productivity, and a new mechanism by which models of biodiversity–ecosystem functioning relationships can be improved.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Tilman, D., Reich, P. B. & Isbell, F. Biodiversity impacts ecosystem productivity as much as resources, disturbance, or herbivory. Proc. Natl Acad. Sci. USA 109, 10394–10397 (2012)
Liang, J . et al. Positive biodiversity-productivity relationship predominant in global forests. Science 354, aaf8957 (2016)
Vandenkoornhuyse, P., Quaiser, A., Duhamel, M., Le Van, A. & Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 206, 1196–1206 (2015)
Vorholt, J. A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 10, 828–840 (2012)
Bringel, F. & Couée, I. Pivotal roles of phyllosphere microorganisms at the interface between plant functioning and atmospheric trace gas dynamics. Front. Microbiol. 6, 486 (2015)
Müller, D. B., Vogel, C., Bai, Y. & Vorholt, J. A. The plant microbiota: systems biology insights and perspectives. Annu. Rev. Genet. 50, 211–234 (2016)
Hautier, Y. et al. Plant ecology. Anthropogenic environmental changes affect ecosystem stability via biodiversity. Science 348, 336–340 (2015)
Allan, E. et al. Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol. Lett. 18, 834–843 (2015)
Loreau, M. & Hector, A. Partitioning selection and complementarity in biodiversity experiments. Nature 412, 72–76 (2001)
Flynn, D. F., Mirotchnick, N., Jain, M., Palmer, M. I. & Naeem, S. Functional and phylogenetic diversity as predictors of biodiversity–ecosystem-function relationships. Ecology 92, 1573–1581 (2011)
O’Connor, M. I. et al. A general biodiversity–function relationship is mediated by trophic level. Oikos 126, 18–31 2016)
Knief, C. et al. Metaproteogenomic analysis of microbial communities in the phyllosphere and rhizosphere of rice. ISME J. 6, 1378–1390 (2012)
Jo, Y. et al. Bacterial communities in the phylloplane of Prunus species. J. Basic Microbiol. 55, 504–508 (2015)
Moyes, A. B. et al. Evidence for foliar endophytic nitrogen fixation in a widely distributed subalpine conifer. New Phytol. 210, 657–668 (2016)
Bodenhausen, N., Bortfeld-Miller, M., Ackermann, M. & Vorholt, J. A. A synthetic community approach reveals plant genotypes affecting the phyllosphere microbiota. PLoS Genet. 10, e1004283 (2014)
Zamioudis, C. & Pieterse, C. M. J. Modulation of host immunity by beneficial microbes. Mol. Plant Microbe Interact. 25, 139–150 (2012)
Khanna, S. et al. A novel microbiome therapeutic increases gut microbial diversity and prevents recurrent Clostridium difficile infection. J. Infect. Dis. 214, 173–181 (2016)
Berendsen, R. L., Pieterse, C. M. & Bakker, P. A. The rhizosphere microbiome and plant health. Trends Plant Sci. 17, 478–486 (2012)
Agler, M. T. et al. Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol. 14, e1002352 (2016)
Ritpitakphong, U. et al. The microbiome of the leaf surface of Arabidopsis protects against a fungal pathogen. New Phytol. 210, 1033–1043 (2016)
Brandl, M. T., Quiñones, B. & Lindow, S. E. Heterogeneous transcription of an indoleacetic acid biosynthetic gene in Erwinia herbicola on plant surfaces. Proc. Natl Acad. Sci. USA 98, 3454–3459 (2001)
Brandl, M. T. & Lindow, S. E. Contribution of indole-3-acetic acid production to the epiphytic fitness of Erwinia herbicola. Appl. Environ. Microbiol. 64, 3256–3263 (1998)
Laforest-Lapointe, I., Messier, C. & Kembel, S. W. Host species identity, site and time drive temperate tree phyllosphere bacterial community structure. Microbiome 4, 27 (2016)
Kembel, S. W. et al. Relationships between phyllosphere bacterial communities and plant functional traits in a neotropical forest. Proc. Natl Acad. Sci. USA 111, 13715–13720 (2014)
Sapijanskas, J., Paquette, A., Potvin, C., Kunert, N. & Loreau, M. Tropical tree diversity enhances light capture through crown plasticity and spatial and temporal niche differences. Ecology 95, 2479–2492 (2014)
Fargione, J. et al. From selection to complementarity: shifts in the causes of biodiversity–productivity relationships in a long-term biodiversity experiment. Proc. R. Soc. B 274, 871–876 (2007)
Poisot, T., Mouquet, N. & Gravel, D. Trophic complementarity drives the biodiversity-ecosystem functioning relationship in food webs. Ecol. Lett. 16, 853–861 (2013)
Wei, F., Hu, X. & Xu, X. Dispersal of Bacillus subtilis and its effect on strawberry phyllosphere microbiota under open field and protection conditions. Sci. Rep. 6, 22611 (2016)
Lau, J. A. & Lennon, J. T. Rapid responses of soil microorganisms improve plant fitness in novel environments. Proc. Natl Acad. Sci. USA 109, 14058–14062 (2012)
Tobner, C. M., Paquette, A., Reich, P. B., Gravel, D. & Messier, C. Advancing biodiversity-ecosystem functioning science using high-density tree-based experiments over functional diversity gradients. Oecologia 174, 609–621 (2014)
Verheyen, K. et al. Contributions of a global network of tree diversity experiments to sustainable forest plantations. Ambio 45, 29–41 (2016)
Tobner, C. M. et al. Functional identity is the main driver of diversity effects in young tree communities. Ecol. Lett. 19, 638–647 (2016)
Laliberté, E. & Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 91, 299–305 (2010)
Lavorel, S. et al. Assessing functional diversity in the field–methodology matters! Funct. Ecol. 22, 134–147 (2008)
Fadrosh, D. W. et al. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2, 6 (2014)
Redford, A. J., Bowers, R. M., Knight, R., Linhart, Y. & Fierer, N. The ecology of the phyllosphere: geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Environ. Microbiol. 12, 2885–2893 (2010)
Zhang, J., Kobert, K., Flouri, T. & Stamatakis, A. PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 30, 614–620 (2014)
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Met. 7, 335–336 (2010)
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010)
DeSantis, T. Z. et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72, 5069–5072 (2006)
Paradis, E ., Claude, J . & Strimmer, K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 (2004)
Kembel, S. W. et al. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464 (2010)
Oksanen, J. et al. The vegan package. Community Ecology Package 10, 631–637 (2007)
R Development Core Team. R: A Language and Environment for Statistical Computing; http://www.R-project.org/ (Vienna, Austria, 2013)
Nakagawa, S. & Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142 (2013)
Johnson, P. C. Extension of Nakagawa & Schielzeth’s R(2)GLMM to random slopes models. Methods Ecol. Evol. 5, 944–946 (2014)
Chave, J. et al. Towards a worldwide wood economics spectrum. Ecol. Lett. 12, 351–366 (2009)
Niinemets, Ü. & Valladares, F. Tolerance to shade, drought and waterlogging of temperate, northern hemisphere trees and shrubs. Ecol. Monogr. 76, 521–547 (2006)
Royal Botanic Gardens Kew. Seed Information Database (SID) version 7.1; http://data.kew.org/sid/ (2008)
Wright, I. J. et al. The worldwide leaf economics spectrum. Nature 428, 821–827 (2004)
Acknowledgements
We are grateful to R. Fréchon, S. Guérard and M. A. Chadid Hernandez for their help in the field, and to J. Shapiro and his laboratory for technical support. We also thank B. Shipley for his help with structural equation modelling techniques used in the manuscript. C. M. Tobner, P. B. Reich and D. Gravel helped in designing the original experiment (IDENT-Montréal) together with A.P. and C.M. The study site is part of McGill University and we much appreciate their support.
Author information
Authors and Affiliations
Contributions
I.L.-L., C.M. and S.W.K. designed the study; C.M. and A.P. established the field experiment; I.L.-L. collected the data; I.L.-L. analysed the data with support from A.P. and S.W.K.; all authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks D. Wardle, S. Lindow, J. Grace and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Identity of the tree host species in each of the 54 combinations at the IDENT experiment in Montreal.
Ab, Abies balsamea; Ap, Acer platanoides; Ar, Acer rubrum; As, Acer saccharum; Ba, Betula alleghaniensis; Bp, Betula papyrifera; Ld, Larix decidua; Ll, Larix laricina; Pa, Picea abies; Pg, Picea glauca; Po, Picea omorika; Pre, Pinus resinosa; Pru, Picea rubens; Pst, Pinus strobus; Psy, Pinus sylvestris; Qro, Quercus robur; Qru, Quercus rubra; Tc, Tilia cordata; To, Thuja occidentalis.
Extended Data Figure 2 Principal component analysis on functional traits community weighted means.
Traits are: maximum photosynthetic capacity (Amass), nitrogen content of leaves (Nmass), leaf longevity (LL), wood density (WD) and leaf mass per area (LMA). Colours represent plot species richness levels (red for one species, orange for two, green for four, and blue for twelve).
Extended Data Figure 3 A priori structural equation model.
Factors are species richness, functional identity, functional diversity and plot microtopography (elevation at plot centre, cm) as determinants of leaf bacterial diversity and plant community productivity. Green boxes indicate exogenous variables (diversity indices and plot microtopography), whereas responses are in yellow for plot-level leaf bacterial diversity and blue for plant community productivity.
Extended Data Figure 4 Alternative structural equation model excluding the link between leaf bacterial diversity and plant community productivity.
After deletion of this link, the path analysis (n = 216, χ2 = 11.906, P = 0.008, df = 3; RMSEA = 0.00, P = 0.044) is unstable and inferior to the model with the leaf bacterial diversity–plant community productivity link included. Green boxes indicate plot-level plant diversity indices; yellow denotes plot-level leaf bacterial diversity; blue denotes plant community productivity. Numbers adjacent to arrows and arrow width indicate the effect size of the relationships and the associated bootstrap P value. +P < 0.1; *P < 0.05; **P < 0.01; ***P < 0.001. Continuous and dashed arrows indicate positive and negative relationships, respectively.
Extended Data Figure 5 Structural equation model of plant diversity and identity explaining leaf bacterial diversity and community composition, as well as plant community productivity (full model tested).
Green boxes indicate plot-level plant diversity indices; yellow denotes plot-level leaf bacterial diversity; orange indicates plot-level leaf bacterial identity; blue denotes for plant community productivity. The covariances between leaf bacterial diversity and the two variables of leaf bacterial community composition were also included in the model.
Extended Data Figure 6 Structural equation model of plant diversity and identity explaining leaf bacterial diversity and community composition, as well as plant community productivity.
The path analysis (n = 216, χ2 = 1.522, P = 0.677, df = 3; RMSEA = 0.00, P = 0.821) explains 38% of the variance in leaf bacterial diversity, 34% and 11% of each components of bacterial identity, and 85% of the variance in plot productivity. Green boxes indicate plot-level plant diversity indices; yellow denotes plot-level leaf bacterial diversity; orange indicates plot-level leaf bacterial identity; blue denotes plant community productivity. Numbers adjacent to arrows and arrow width indicate the effect size of the relationships and the associated bootstrap P value. +P < 0.1; *P < 0.05; **P < 0.01; ***P < 0.001. Continuous and dashed arrows indicate positive and negative relationships, respectively. The covariances between leaf bacterial diversity and the two variables of leaf bacterial community composition were also included in the model.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Laforest-Lapointe, I., Paquette, A., Messier, C. et al. Leaf bacterial diversity mediates plant diversity and ecosystem function relationships. Nature 546, 145–147 (2017). https://doi.org/10.1038/nature22399
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature22399
This article is cited by
-
Selective regulation of endophytic bacteria and gene expression in soybean by water-soluble humic materials
Environmental Microbiome (2024)
-
Forest top canopy bacterial communities are influenced by elevation and host tree traits
Environmental Microbiome (2024)
-
Disentangling direct vs indirect effects of microbiome manipulations in a habitat-forming marine holobiont
npj Biofilms and Microbiomes (2024)
-
Interplay of biotic and abiotic factors shapes tree seedling growth and root-associated microbial communities
Communications Biology (2024)
-
Analysis of diversity and function of epiphytic bacterial communities associated with macrophytes using a metagenomic approach
Microbial Ecology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.