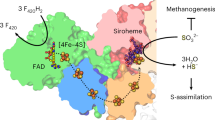
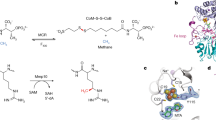
Abstract
Methane biogenesis in methanogens is mediated by methyl-coenzyme M reductase, an enzyme that is also responsible for the utilization of methane through anaerobic methane oxidation. The enzyme uses an ancillary factor called coenzyme F430, a nickel-containing modified tetrapyrrole that promotes catalysis through a methyl radical/Ni(ii)-thiolate intermediate. However, it is unclear how coenzyme F430 is synthesized from the common primogenitor uroporphyrinogen iii, incorporating 11 steric centres into the macrocycle, although the pathway must involve chelation, amidation, macrocyclic ring reduction, lactamization and carbocyclic ring formation. Here we identify the proteins that catalyse the biosynthesis of coenzyme F430 from sirohydrochlorin, termed CfbA–CfbE, and demonstrate their activity. The research completes our understanding of how the repertoire of tetrapyrrole-based pigments are constructed, permitting the development of recombinant systems to use these metalloprosthetic groups more widely.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
01 March 2017
In Fig. 1a, a missing bond was added to ring F.
References
Ragsdale, S. W. Biochemistry of methyl-coenzyme M reductase: the nickel metalloenzyme that catalyzes the final step in synthesis and the first step in anaerobic oxidation of the greenhouse gas methane. Met. Ions Life Sci . 14, 125–145 (2014)
Thauer, R. K. Biochemistry of methanogenesis: a tribute to Marjory Stephenson. 1998 Marjory Stephenson Prize Lecture. Microbiology 144, 2377–2406 (1998)
Raghoebarsing, A. A. et al. A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 440, 918–921 (2006)
Scheller, S., Goenrich, M., Boecher, R., Thauer, R. K. & Jaun, B. The key nickel enzyme of methanogenesis catalyses the anaerobic oxidation of methane. Nature 465, 606–608 (2010)
Shima, S. et al. Structure of a methyl-coenzyme M reductase from Black Sea mats that oxidize methane anaerobically. Nature 481, 98–101 (2011)
Krüger, M. et al. A conspicuous nickel protein in microbial mats that oxidize methane anaerobically. Nature 426, 878–881 (2003)
Ermler, U., Grabarse, W., Shima, S., Goubeaud, M. & Thauer, R. K. Crystal structure of methyl-coenzyme M reductase: the key enzyme of biological methane formation. Science 278, 1457–1462 (1997)
Wongnate, T. & Ragsdale, S. W. The reaction mechanism of methyl-coenzyme M reductase: how an enzyme enforces strict binding order. J. Biol. Chem. 290, 9322–9334 (2015)
Wongnate, T. et al. The radical mechanism of biological methane synthesis by methyl-coenzyme M reductase. Science 352, 953–958 (2016)
Thauer, R. K. & Bonacker, L. G. Biosynthesis of coenzyme F430, a nickel porphinoid involved in methanogenesis. Ciba Found. Symp. 180, 210–222, discussion 222–227 (1994)
Gilles, H. & Thauer, R. K. Uroporphyrinogen iii, an intermediate in the biosynthesis of the nickel-containing factor F430 in Methanobacterium thermoautotrophicum. Eur. J. Biochem . 135, 109–112 (1983)
Warren, M. J. & Scott, A. I. Tetrapyrrole assembly and modification into the ligands of biologically functional cofactors. Trends Biochem. Sci . 15, 486–491 (1990)
Färber, G. et al. Coenzyme F430 from methanogenic bacteria—complete assignment of configuration based on an X-ray-analysis of 12,13-diepi-F430 pentamethyl ester and on NMR-spectroscopy. Helv. Chim. Acta 74, 697–716 (1991)
Mucha, H., Keller, E., Weber, H., Lingens, F. & Trosch, W. Sirohydrochlorin, a precursor of factor-F430 biosynthesis in Methanobacterium thermoautotrophicum. FEBS Lett . 190, 169–171 (1985)
Pfaltz, A., Kobelt, A., Hüster, R. & Thauer, R. K. Biosynthesis of coenzyme F430 in methanogenic bacteria. Identification of 15,173-seco-F430-173-acid as an intermediate. Eur. J. Biochem . 170, 459–467 (1987)
Brindley, A. A., Raux, E., Leech, H. K., Schubert, H. L. & Warren, M. J. A story of chelatase evolution: identification and characterization of a small 13-15-kDa “ancestral” cobaltochelatase (CbiXS) in the archaea. J. Biol. Chem. 278, 22388–22395 (2003)
Yan, Y. et al. Crystal structure of Escherichia coli UDPMurNAc-tripeptide d-alanyl-d-alanine-adding enzyme (MurF) at 2.3 A resolution. J. Mol. Biol. 304, 435–445 (2000)
Staples, C. R. et al. Expression and association of group IV nitrogenase NifD and NifH homologs in the non-nitrogen-fixing archaeon Methanocaldococcus jannaschii. J. Bacteriol . 189, 7392–7398 (2007)
Warren, M. J., Raux, E., Schubert, H. L. & Escalante-Semerena, J. C. The biosynthesis of adenosylcobalamin (vitamin B12). Nat. Prod. Rep . 19, 390–412 (2002)
Chivers, P. T. Nickel recognition by bacterial importer proteins. Metallomics 7, 590–595 (2015)
Eitinger, T. & Mandrand-Berthelot, M. A. Nickel transport systems in microorganisms. Arch. Microbiol . 173, 1–9 (2000)
Mulrooney, S. B. & Hausinger, R. P. Nickel uptake and utilization by microorganisms. FEMS Microbiol. Rev . 27, 239–261 (2003)
Chivers, P. T., Benanti, E. L., Heil-Chapdelaine, V., Iwig, J. S. & Rowe, J. L. Identification of Ni-(l-His)2 as a substrate for NikABCDE-dependent nickel uptake in Escherichia coli. Metallomics 4, 1043–1050 (2012)
Waldron, K. J., Rutherford, J. C., Ford, D. & Robinson, N. J. Metalloproteins and metal sensing. Nature 460, 823–830 (2009)
Deery, E. et al. An enzyme-trap approach allows isolation of intermediates in cobalamin biosynthesis. Nat. Chem. Biol . 8, 933–940 (2012)
Bröcker, M. J. et al. Crystal structure of the nitrogenase-like dark operative protochlorophyllide oxidoreductase catalytic complex (ChlN/ChlB)2 . J. Biol. Chem. 285, 27336–27345 (2010)
Muraki, N. et al. X-ray crystal structure of the light-independent protochlorophyllide reductase. Nature 465, 110–114 (2010)
Fässler, A. et al. Preparation and properties of some hydrocorphinoid nickel(ii)-complexes. Helv. Chim. Acta 65, 812–827 (1982)
Schlingmann, G., Dresow, B., Ernst, L. & Koppenhagen, V. B. The structure of yellow metal-free and cobalt-containing corrinoids. Liebigs Ann. Chem . 2061–2066 (1981)
Schlingmann, G., Dresow, B., Koppenhagen, V. B., Becker, W. & Sheldrick, W. S. Structure of yellow metal-free and yellow cobalt-containing corrinoids. Angew. Chem. Int. Edn Engl . 19, 321–322 (1980)
Lee, C. C., Ribbe, M. W. & Hu, Y. Cleaving the N,N triple bond: the transformation of dinitrogen to ammonia by nitrogenases. Met. Ions Life Sci . 14, 147–176 (2014)
Won, H., Olson, K. D., Wolfe, R. S. & Summers, M. F. 2-dimensional NMR-studies of native coenzyme-F430 . J. Am. Chem. Soc. 112, 2178–2184 (1990)
Battersby, A. R. How nature builds the pigments of life: the conquest of vitamin B12 . Science 264, 1551–1557 (1994)
Moore, S. J. et al. Elucidation of the anaerobic pathway for the corrin component of cobalamin (vitamin B12). Proc. Natl Acad. Sci. USA 110, 14906–14911 (2013)
Bali, S. et al. Molecular hijacking of siroheme for the synthesis of heme and d1 heme. Proc. Natl Acad. Sci. USA 108, 18260–18265 (2011)
Dailey, H. A., Gerdes, S., Dailey, T. A., Burch, J. S. & Phillips, J. D. Noncanonical coproporphyrin-dependent bacterial heme biosynthesis pathway that does not use protoporphyrin. Proc. Natl Acad. Sci. USA 112, 2210–2215 (2015)
McGoldrick, H. M. et al. Identification and characterization of a novel vitamin B12 (cobalamin) biosynthetic enzyme (CobZ) from Rhodobacter capsulatus, containing flavin, heme, and Fe-S cofactors. J. Biol. Chem. 280, 1086–1094 (2005)
Flühe, L. et al. The radical SAM enzyme AlbA catalyzes thioether bond formation in subtilosin A. Nat. Chem. Biol . 8, 350–357 (2012)
Kühner, M. et al. The alternative route to heme in the methanogenic archaeon Methanosarcina barkeri. Archaea 2014, 327637 (2014)
Schubert, H. L. et al. The structure of Saccharomyces cerevisiae Met8p, a bifunctional dehydrogenase and ferrochelatase. EMBO J . 21, 2068–2075 (2002)
Bartolommei, G., Moncelli, M. R. & Tadini-Buoninsegni, F. A method to measure hydrolytic activity of adenosinetriphosphatases (ATPases). PLoS One 8, e58615 (2013)
Schanda, P., Kupce, E. & Brutscher, B. SOFAST-HMQC experiments for recording two-dimensional heteronuclear correlation spectra of proteins within a few seconds. J. Biomol. NMR 33, 199–211 (2005)
Acknowledgements
We thank M. Höninger, T. Schnitzer and J. Streif for conducting initial experiments with CfbA and CfbC/CfbD. We thank R. Thauer and S. Shima for the gift of the F430 standard. This work was supported by grants from the Boehringer Ingelheim Foundation (Exploration Grant) and the Deutsche Forschungsgemeinschaft (LA2412/6-1) to G.L. and from the Biotechnology and Biological Sciences Research Council (BBSRC; 68/B19356 and BB/I012079) to M.J.W.
Author information
Authors and Affiliations
Contributions
S.J.M., S.T.S., C.S., E.D., A.D.L., J.V.R., S.B. and C.B. all undertook aspects of the experimental work, cloning, protein purification and enzyme assays, and helped with the interpretation of the data. P.T.C. provided the nixA clone and helped design the nickel uptake system. M.J.H., S.J.M. and A.D.L. designed and interpreted the NMR experiments and S.E.J.R., together with S.J.M. and A.D.L., provided the EPR data. S.J.M., M.J.W. and G.L. designed the experiments and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Nickel chelatase activity of CfbA.
a, b, In vitro activity assay of CfbA. Purified CfbA was incubated with sirohydrochlorin and NiSO4 at 37 °C (a). The insertion of nickel was monitored by UV/Vis absorption spectroscopy every 15 min. When CfbA was omitted from the assay mixture (b), no nickel insertion was observed. c, In vivo activity of CfbA. Cell pellets of E. coli cells transformed with either pETcoco-2-cobA-sirC-cfbA or pETcoco-2-cobA-sirC-cfbA-nixA grown in the presence of nickel.
Extended Data Figure 2 Amidotransferase activity of CfbE.
a, In vivo activity of CfbE. E. coli cells transformed with pETcoco-2-cobA-sirC-cfbA-nixA and pET14b-cfbE and grown in the presence of nickel produce a dark violet pigment that co-purifies with CfbE during IMAC. b, c, 15N labelling of nickel-sirohydrochlorin a,c-diamide. b, Reverse-phase HPLC chromatogram of nickel-sirohydrochlorin substrate, m/z = 919 (i); unlabelled nickel-sirohydrochlorin a,c-diamide, m/z = 917 (ii); and 15N labelled nickel-sirohydrochlorin a,c-diamide, m/z = 919 (iii). c, 1H–15N HSQC of an ATP limited titration with nickel-sirohydrochlorin, CfbE and 15NH3. The a and c amide groups increase proportionally in intensity as the level of ATP increases.
Extended Data Figure 3 NMR characterization of Ni2+-sirohydrochlorin a,c-diamide.
a, b, 1H–13C HSQC (a) and 1H–15N HSQC (b) of 4 mM Ni2+-sirohydrochlorin a,c-diamide in D2O.
Extended Data Figure 4 Steady-state kinetics of the M. barkeri CfbE amidotransferase with glutamine or ATP as a variable.
a, 1 mM glutamine with ATP varied between 0.05 and 1.5 mM ATP. b, 0.5 mM ATP with glutamine varied between 0.05 and 10 mM. Fixed conditions: buffer B, 20 °C, 2.5 μM M. barkeri CfbE, 25 μM nickel-sirohydrochlorin, 5 mM MgCl2. The mean and error bars were calculated from 3 technical repeats.
Extended Data Figure 5 Characterization of the CfbC/CfbD assay reaction products by mass spectrometry after HPLC separation.
a, Mass spectrum with the isotopic pattern of the reaction product after 1.5 h of incubation measured in positive ion mode. b, Mass spectrum with the isotopic pattern of the reaction product after 22 h of incubation measured in positive ion mode.
Extended Data Figure 6 NMR characterization of seco-F430.
a, b, 1H–13C HSQC (a) and 1H–15N HSQC (b) of 4 mM seco-F430 in D2O.
Extended Data Figure 7 Characterization of the CfbB assay reaction products.
a, UV/Vis absorption spectrum of an F430 standard in 0.01% formic acid/acetonitrile. b, CfbB assay with Ni2+-hexahydrosirohydrochlorin a,c-diamide as the substrate. Mass spectrum with the isotopic pattern of the reaction product after 2 h of incubation measured in positive ion mode after HPLC separation. c, CfbB assay with seco-F430 as the substrate. Mass spectrum with the isotopic pattern of the reaction product after 22 h of incubation measured in positive ion mode after HPLC separation.
Extended Data Figure 8 NMR characterization of F430 synthesized by CfbB.
1H–13C HSQC and 1H–15N HSQC of F430 in TFE-d3.
Extended Data Figure 9 Proposed mechanism for the reaction catalysed by CfbB.
Initially, CfbB promotes the ATP-dependent phosphorylation of the propionic acid side chain on ring D of seco-F430. This activated side chain is then able to undergo cyclisation to form ring F and thereby generate coenzyme F430.
Supplementary information
Supplementary Table 1
NMR chemical shift assignments for Ni2+-sirohydrochlorin a,c-diamide 50 mM KPi pH 8.0 (D2O). (XLSX 12 kb)
Supplementary Table 2
NMR chemical shift assignments for seco-F430 in D2O (XLSX 12 kb)
Supplementary Table 3
NMR chemical shift assignments for F430 in TFE-d3 (XLSX 9 kb)
Rights and permissions
About this article
Cite this article
Moore, S., Sowa, S., Schuchardt, C. et al. Elucidation of the biosynthesis of the methane catalyst coenzyme F430. Nature 543, 78–82 (2017). https://doi.org/10.1038/nature21427
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature21427
This article is cited by
-
Influence of sulfide on diazotrophic growth of the methanogen Methanococcus maripaludis and its implications for the origin of nitrogenase
Communications Biology (2023)
-
Bovine host genome acts on rumen microbiome function linked to methane emissions
Communications Biology (2022)
-
Groundwater Elusimicrobia are metabolically diverse compared to gut microbiome Elusimicrobia and some have a novel nitrogenase paralog
The ISME Journal (2020)
-
An evolving view of methane metabolism in the Archaea
Nature Reviews Microbiology (2019)
-
Methyl-coenzyme M reductase-dependent endogenous methane enhances plant tolerance against abiotic stress and alters ABA sensitivity in Arabidopsis thaliana
Plant Molecular Biology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.