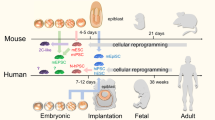
Abstract
Chimaeras are both monsters of the ancient imagination and a long-established research tool. Recent advances, particularly those dealing with the identification and generation of various kinds of stem cells, have broadened the repertoire and utility of mammalian interspecies chimaeras and carved out new paths towards understanding fundamental biology as well as potential clinical applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ito, R., Takahashi, T., Katano, I. & Ito, M. Current advances in humanized mouse models. Cell. Mol. Immunol. 9, 208–214 (2012)
Le Douarin, N. M. The ontogeny of the neural crest in avian embryo chimaeras. Nature 286, 663–669 (1980)
Douarin, N. L. & Kalcheim, C. The Neural Crest (Cambridge Univ. Press, 1982)
Gardner, R. L. & Johnson, M. H. Investigation of early mammalian development using interspecific chimaeras between rat and mouse. Nature 246, 86–89 (1973)
Rossant, J. & Frels, W. I. Interspecific chimeras in mammals: successful production of live chimeras between Mus musculus and Mus caroli. Science 208, 419–421 (1980). A pioneering study reporting the first viable interspecies chimaeras generated by injection of M. caroli ICMs into M. musculus blastocysts
Rossant, J., Vijh, M., Siracusa, L. D. & Chapman, V. M. Identification of embryonic cell lineages in histological sections of M. musculus in-equilibrium M. caroli chimaeras. J. Embryol. Exp. Morphol. 73, 179–191 (1983)
Fehilly, C. B., Willadsen, S. M. & Tucker, E. M. Interspecific chimaerism between sheep and goat. Nature 307, 634–636 (1984)
Williams, T. J., Munro, R. K. & Shelton, J. N. Production of interspecies chimeric calves by aggregation of Bos indicus and Bos taurus demi-embryos. Reprod. Fertil. Dev. 2, 385–394 (1990)
Rossant, J., Croy, B. A., Chapman, V. M., Siracusa, L. & Clark, D. A. Interspecific chimeras in mammals: a new experimental system. J. Anim. Sci. 55, 1241–1248 (1982)
Rossant, J., Mauro, V. M. & Croy, B. A. Importance of trophoblast genotype for survival of interspecific murine chimeras. J. Embryol. Exp. Morphol. 69, 141–149 (1982)
Wu, J. & Izpisua Belmonte, J. C. Dynamic pluripotent stem cell states and their applications. Cell Stem Cell 17, 509–525 (2015)
Xiang, A. P. et al. Extensive contribution of embryonic stem cells to the development of an evolutionarily divergent host. Hum. Mol. Genet. 17, 27–37 (2008). The first study reporting PS-cell-derived viable interspecies chimaeras between two distant rodent species
Han, X. et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12, 342–353 (2013)
Ying, Q.-L. et al. The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 (2008). A landmark paper describing the pluripotent ground state and how the ground state culture ultimately enabled derivation of genuine ES cells from rat blastocysts (see also refs 15, 16)
Buehr, M. et al. Capture of authentic embryonic stem cells from rat blastocysts. Cell 135, 1287–1298 (2008)
Li, P. et al. Germline competent embryonic stem cells derived from rat blastocysts. Cell 135, 1299–1310 (2008)
Kobayashi, T. et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell 142, 787–799 (2010). A landmark paper reporting PS-cell-derived viable mouse–rat interspecies chimaeras and demonstrating the first proof-of-concept of interspecies blastocyst complementation
Isotani, A., Hatayama, H., Kaseda, K., Ikawa, M. & Okabe, M. Formation of a thymus from rat ES cells in xenogeneic nude mouse↔rat ES chimeras. Genes Cells 16, 397–405 (2011)
Thomson, J. A. et al. Isolation of a primate embryonic stem cell line. Proc. Natl Acad. Sci. USA 92, 7844–7848 (1995)
Sasaki, E. et al. Establishment of novel embryonic stem cell lines derived from the common marmoset (Callithrix jacchus). Stem Cells 23, 1304–1313 (2005)
Simerly, C. R. et al. Establishment and characterization of baboon embryonic stem cell lines: an Old World Primate model for regeneration and transplantation research. Stem Cell Res. (Amst.) 2, 178–187 (2009)
Thomson, J. A. et al. Pluripotent cell lines derived from common marmoset (Callithrix jacchus) blastocysts. Biol. Reprod. 55, 254–259 (1996)
Thomson, J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998)
Reubinoff, B. E., Pera, M. F., Fong, C. Y., Trounson, A. & Bongso, A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat. Biotechnol. 18, 399–404 (2000)
Tachibana, M. et al. Generation of chimeric rhesus monkeys. Cell 148, 285–295 (2012)
Simerly, C. et al. Interspecies chimera between primate embryonic stem cells and mouse embryos: monkey ESCs engraft into mouse embryos, but not post-implantation fetuses. Stem Cell Res. (Amst.) 7, 28–40 (2011)
James, D., Noggle, S. A., Swigut, T. & Brivanlou, A. H. Contribution of human embryonic stem cells to mouse blastocysts. Dev. Biol. 295, 90–102 (2006)
Nichols, J. & Smith, A. Naive and primed pluripotent states. Cell Stem Cell 4, 487–492 (2009)
Boroviak, T., Loos, R., Bertone, P., Smith, A. & Nichols, J. The ability of inner-cell-mass cells to self-renew as embryonic stem cells is acquired following epiblast specification. Nat. Cell Biol. 16, 516–528 (2014)
Brons, I. G. M. et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448, 191–195 (2007)
Tesar, P. J. et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448, 196–199 (2007)
Huang, Y., Osorno, R., Tsakiridis, A. & Wilson, V. In vivo differentiation potential of epiblast stem cells revealed by chimeric embryo formation. Cell Reports 2, 1571–1578 (2012)
Kojima, Y. et al. The transcriptional and functional properties of mouse epiblast stem cells resemble the anterior primitive streak. Cell Stem Cell 14, 107–120 (2014)
Wu, J. et al. An alternative pluripotent state confers interspecies chimaeric competency. Nature 521, 316–321 (2015)
Mascetti, V. L. & Pedersen, R. A. Human–mouse chimerism validates human stem cell pluripotency. Cell Stem Cell 18, 67–72 (2016). Refs 34, 35 are two pioneering studies that demonstrated functional engraftment of primed human PS cells into gastrula-stage mouse embryos
Wu, J. & Izpisua Belmonte, J. C. Stem cells: a renaissance in human biology research. Cell 165, 1572–1585 (2016)
Mascetti, V. L. & Pedersen, R. A. Contributions of mammalian chimeras to pluripotent stem cell research. Cell Stem Cell 19, 163–175 (2016)
Hanna, J. et al. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc. Natl Acad. Sci. USA 107, 9222–9227 (2010). A pioneering study demonstrating that a naive human pluripotent state, resembling that of mouse ES cells, could be stabilized in culture
Chan, Y.-S. et al. Induction of a human pluripotent state with distinct regulatory circuitry that resembles preimplantation epiblast. Cell Stem Cell 13, 663–675 (2013). Refs 39, 41, 42, 43, 46 are examples of the first studies describing different culture conditions for naive human ES cells
Duggal, G. et al. Alternative routes to induce naïve pluripotency in human embryonic stem cells. Stem Cells 33, 2686–2698 (2015)
Gafni, O. et al. Derivation of novel human ground state naive pluripotent stem cells. Nature 504, 282–286 (2013)
Takashima, Y. et al. Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell 158, 1254–1269 (2014)
Theunissen, T. W. et al. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 15, 471–487 (2014)
Theunissen, T. W. et al. Molecular criteria for defining the naive human pluripotent state. Cell Stem Cell 19, 502–515 (2016)
Wang, J. et al. Primate-specific endogenous retrovirus-driven transcription defines naive-like stem cells. Nature 516, 405–409 (2014)
Ware, C. B. et al. Derivation of naive human embryonic stem cells. Proc. Natl Acad. Sci. USA 111, 4484–4489 (2014)
Guo, G. et al. Naive pluripotent stem cells derived directly from isolated cells of the human inner cell mass. Stem Cell Rep. 6, 437–446 (2016)
Pera, M. F. In search of naivety. Cell Stem Cell 15, 543–545 (2014)
Huang, K., Maruyama, T. & Fan, G. The naive state of human pluripotent stem cells: a synthesis of stem cell and preimplantation embryo transcriptome analyses. Cell Stem Cell 15, 410–415 (2014)
Masaki, H. et al. Interspecific in vitro assay for the chimera-forming ability of human pluripotent stem cells. Development 142, 3222–3230 (2015)
Chen, Y. et al. Generation of cynomolgus monkey chimeric fetuses using embryonic stem cells. Cell Stem Cell 17, 116–124 (2015)
Fang, R. et al. Generation of naive induced pluripotent stem cells from rhesus monkey fibroblasts. Cell Stem Cell 15, 488–496 (2014)
Dupin, E. & Sommer, L. Neural crest progenitors and stem cells: from early development to adulthood. Dev. Biol. 366, 83–95 (2012)
Jaenisch, R. Mammalian neural crest cells participate in normal embryonic development on microinjection into post-implantation mouse embryos. Nature 318, 181–183 (1985)
Huszar, D., Sharpe, A. & Jaenisch, R. Migration and proliferation of cultured neural crest cells in W mutant neural crest chimeras. Development 112, 131–141 (1991)
Cohen, M. A. et al. Human neural crest cells contribute to coat pigmentation in interspecies chimeras after in utero injection into mouse embryos. Proc. Natl Acad. Sci. USA 113, 1570–1575 (2016)
Brüstle, O. et al. Chimeric brains generated by intraventricular transplantation of fetal human brain cells into embryonic rats. Nat. Biotechnol. 16, 1040–1044 (1998)
Keyoung, H. M. et al. High-yield selection and extraction of two promoter-defined phenotypes of neural stem cells from the fetal human brain. Nat. Biotechnol. 19, 843–850 (2001)
Goyama, S., Wunderlich, M. & Mulloy, J. C. Xenograft models for normal and malignant stem cells. Blood 125, 2630–2640 (2015)
Zanjani, E. D., Almeida-Porada, G., Livingston, A. G., Flake, A. W. & Ogawa, M. Human bone marrow CD34− cells engraft in vivo and undergo multilineage expression that includes giving rise to CD34+ cells. Exp. Hematol. 26, 353–360 (1998)
Fujiki, Y. et al. Successful multilineage engraftment of human cord blood cells in pigs after in utero transplantation. Transplantation 75, 916–922 (2003)
Muotri, A. R., Nakashima, K., Toni, N., Sandler, V. M. & Gage, F. H. Development of functional human embryonic stem cell-derived neurons in mouse brain. Proc. Natl Acad. Sci. USA 102, 18644–18648 (2005)
Chuma, S. et al. Spermatogenesis from epiblast and primordial germ cells following transplantation into postnatal mouse testis. Development 132, 117–122 (2005)
Leitch, H. G. et al. On the fate of primordial germ cells injected into early mouse embryos. Dev. Biol. 385, 155–159 (2014)
Clarke, D. L. et al. Generalized potential of adult neural stem cells. Science 288, 1660–1663 (2000)
Jiang, Y. et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418, 41–49 (2002)
Geiger, H., Sick, S., Bonifer, C. & Müller, A. M. Globin gene expression is reprogrammed in chimeras generated by injecting adult hematopoietic stem cells into mouse blastocysts. Cell 93, 1055–1065 (1998)
Harder, F., Henschler, R., Junghahn, I., Lamers, M. C. & Müller, A. M. Human hematopoiesis in murine embryos after injecting human cord blood-derived hematopoietic stem cells into murine blastocysts. Blood 99, 719–721 (2002)
Wilson, A. & Trumpp, A. Bone-marrow haematopoietic-stem-cell niches. Nat. Rev. Immunol. 6, 93–106 (2006)
Brinster, R. L. & Zimmermann, J. W. Spermatogenesis following male germ-cell transplantation. Proc. Natl Acad. Sci. USA 91, 11298–11302 (1994)
Hayashi, K., Ohta, H., Kurimoto, K., Aramaki, S. & Saitou, M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 146, 519–532 (2011)
Morata, G. & Ripoll, P. Minutes: mutants of Drosophila autonomously affecting cell division rate. Dev. Biol. 42, 211–221 (1975)
Amoyel, M. & Bach, E. A. Cell competition: how to eliminate your neighbours. Development 141, 988–1000 (2014)
Dejosez, M., Ura, H., Brandt, V. L. & Zwaka, T. P. Safeguards for cell cooperation in mouse embryogenesis shown by genome-wide cheater screen. Science 341, 1511–1514 (2013)
Masaki, H. et al. Inhibition of apoptosis overcomes stage-related compatibility barriers to chimera formation in mouse embryos. Cell Stem Cell 19, 587–592 (2016)
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).This landmark study established that somatic cells could be induced to PS cells by defined transcription factors
Usui, J. et al. Generation of kidney from pluripotent stem cells via blastocyst complementation. Am. J. Pathol. 180, 2417–2426 (2012)
Matsunari, H. et al. Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. Proc. Natl Acad. Sci. USA 110, 4557–4562 (2013)
Rashid, T., Kobayashi, T. & Nakauchi, H. Revisiting the flight of Icarus: making human organs from PS cells with large animal chimeras. Cell Stem Cell 15, 406–409 (2014)
Kobayashi, T., Kato-Itoh, M. & Nakauchi, H. Targeted organ generation using Mixl1-inducible mouse pluripotent stem cells in blastocyst complementation. Stem Cells Dev. 24, 182–189 (2015)
Li, Z. et al. 3D culture supports long-term expansion of mouse and human nephrogenic progenitors. Cell Stem Cell 19, 516–529 (2016)
Kuehn, M. R., Bradley, A., Robertson, E. J. & Evans, M. J. A potential animal model for Lesch–Nyhan syndrome through introduction of HPRT mutations into mice. Nature 326, 295–298 (1987)
Sen, S. Brownback. The Human Chimera Prohibition Act of 2005, S.659 (109th Congress, 2005–2006)
Greely, H. T., Cho, M. K., Hogle, L. F. & Satz, D. M. Thinking about the human neuron mouse. Am. J. Bioeth. 7, 27–40 (2007)
Hyun, I. et al. Ethical standards for human-to-animal chimera experiments in stem cell research. Cell Stem Cell 1, 159–163 (2007)
Hermerén, G. Ethical considerations in chimera research. Development 142, 3–5 (2015)
Hyun, I. From naïve pluripotency to chimeras: a new ethical challenge? Development 142, 6–8 (2015)
Robert, J. S. & Baylis, F. Crossing species boundaries. Am. J. Bioeth. 3, 1–13 (2003)
Karpowicz, P., Cohen, C. B. & van der Kooy, D. It is ethical to transplant human stem cells into nonhuman embryos. Nat. Med. 10, 331–335 (2004)
Karpowicz, P., Cohen, C. B. & van der Kooy, D. Developing human-nonhuman chimeras in human stem cell research: ethical issues and boundaries. Kennedy Inst. Ethics J. 15, 107–134 (2005)
Streiffer, R. in The Stanford Encyclopedia of Philosophy (ed. Zalta, E. N. ) http://plato.stanford.edu/entries/chimeras/ (2009)
Greely, H. T. in Oxford Handbook of Animal Ethics (eds Beauchamp, T. & Frey, R.G. ) (Oxford Univ. Press, 2011)
Johnston, J. & Eliot, C. Chimeras and “human dignity”. Am. J. Bioeth. 3, W6–W8 (2003)
Palacios-González, C. Human dignity and the creation of human–nonhuman chimeras. Med. Health Care Philos. 18, 487–499 (2015)
United States National Institutes of Health. Special Notice: Moratorium on Certain Fetal Tissue Research. NIH Guide for Grants and Contracts. (Special notice, 8 May 1988)
United States National Institutes of Health Revitalization Act of 1993 Public Law 103-43 (1993)
Sherley v. Sebelius, 689 F.3d 776 (D.C. Circuit 2012)
Committee on Guidelines for Human Embryonic Stem Cell Research. Guidelines for Human Embryonic Stem Cell Research (National Academies Press, 2005)
International Society for Stem Cell Research. Guidelines for the Conduct of Human Embryonic Stem Cell Researchhttp://www.isscr.org/home/publications/guide-clintrans (2006)
International Society for Stem Cell Research. Guidelines for Stem Cell Science and Clinical Translationhttp://www.isscr.org/home/publications/2016-guidelines (2016)
Academy of Medical Sciences. Animals Containing Human Materialshttps://issuu.com/acmedsci/docs/animals_containing_human_material. (2011)
Greene, M. et al. Ethics: moral issues of human-non-human primate neural grafting. Science 309, 385–386 (2005)
Palacios-González, C. Ethical aspects of creating human-nonhuman chimeras capable of human gamete production and human pregnancy. Monash Bioeth. Rev. 33, 181–202 (2015)
United States National Institutes of Health. NIH Research Involving Introduction of Human Pluripotent Cells into Non-Human Vertebrate Animal Pre-Gastrulation Embryos. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-158.html (2015)
United States National Institutes of Health. Next Steps on Research Using Animal Embryos Containing Human Cells. http://osp.od.nih.gov/under-the-poliscope/2016/08/next-steps-research-using-animal-embryos-containing-human-cells (2016)
Reardon, S. US agency to lift ban on funding human–animal hybrids. Nature 536, 135 (2016)
Acknowledgements
We would like to thank all our laboratory members and collaborators, including the teams of J. M. Campistol, P. Guillen, E. Martinez and P. Ross for their comments and dedicated work that have greatly contributed to the ideas presented here. H.N. was supported by the California Institute of Regenerative Medicine (CIRM), Japan Agency for Medical Research and Development (AMED) and Takeda Pharmaceuticals International, Inc. J.C.I.B. was supported by the G. Harold and Leila Y. Mathers Charitable Foundation, The Moxie Foundation, Fundación Dr. Pedro Guillen and UCAM. R.J. was supported by grants from the NIH (HD 045022, R37-CA084198, 1R01NS088538-01).
Author information
Authors and Affiliations
Contributions
J.W., H.G., R.J., H.N., J.R. and J.C.I.B conceived the study and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks S. Goldman, I. Hyun and M. A. Surani for their contribution to the peer review of this work.
Rights and permissions
About this article
Cite this article
Wu, J., Greely, H., Jaenisch, R. et al. Stem cells and interspecies chimaeras. Nature 540, 51–59 (2016). https://doi.org/10.1038/nature20573
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature20573
This article is cited by
-
Derivation of transgene-free bat induced pluripotent stem cells amenable to chimera formation in mice, pigs, and chicks
Cell Discovery (2023)
-
Symmetric inheritance of parental histones contributes to safeguarding the fate of mouse embryonic stem cells during differentiation
Nature Genetics (2023)
-
Why it is important to study human–monkey embryonic chimeras in a dish
Nature Methods (2022)
-
Liver development is restored by blastocyst complementation of HHEX knockout in mice and pigs
Stem Cell Research & Therapy (2021)
-
Generation of mouse–human chimeric embryos
Nature Protocols (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.