Abstract
Cellular activity in the brain depends on the high energetic support provided by mitochondria, the cell organelles which use energy sources to generate ATP1,2,3,4. Acute cannabinoid intoxication induces amnesia in humans and animals5,6, and the activation of type-1 cannabinoid receptors present at brain mitochondria membranes (mtCB1) can directly alter mitochondrial energetic activity7,8,9. Although the pathological impact of chronic mitochondrial dysfunctions in the brain is well established1,2, the involvement of acute modulation of mitochondrial activity in high brain functions, including learning and memory, is unknown. Here, we show that acute cannabinoid-induced memory impairment in mice requires activation of hippocampal mtCB1 receptors. Genetic exclusion of CB1 receptors from hippocampal mitochondria prevents cannabinoid-induced reduction of mitochondrial mobility, synaptic transmission and memory formation. mtCB1 receptors signal through intra-mitochondrial Gαi protein activation and consequent inhibition of soluble-adenylyl cyclase (sAC). The resulting inhibition of protein kinase A (PKA)-dependent phosphorylation of specific subunits of the mitochondrial electron transport system eventually leads to decreased cellular respiration. Hippocampal inhibition of sAC activity or manipulation of intra-mitochondrial PKA signalling or phosphorylation of the Complex I subunit NDUFS2 inhibit bioenergetic and amnesic effects of cannabinoids. Thus, the G protein-coupled mtCB1 receptors regulate memory processes via modulation of mitochondrial energy metabolism. By directly linking mitochondrial activity to memory formation, these data reveal that bioenergetic processes are primary acute regulators of cognitive functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mattson, M. P., Gleichmann, M. & Cheng, A. Mitochondria in neuroplasticity and neurological disorders. Neuron 60, 748–766 (2008)
Sheng, Z. H. & Cai, Q. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat. Rev. Neurosci. 13, 77–93 (2012)
Rangaraju, V., Calloway, N. & Ryan, T. A. Activity-driven local ATP synthesis is required for synaptic function. Cell 156, 825–835 (2014)
Attwell, D. & Laughlin, S. B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 21, 1133–1145 (2001)
Broyd, S. J., van Hell, H. H., Beale, C., Yücel, M. & Solowij, N. Acute and chronic effects of cannabinoids on human cognition—a systematic review. Biol. Psychiatry 79, 557–567 (2016)
Marsicano, G. & Lafenêtre, P. Roles of the endocannabinoid system in learning and memory. Curr. Top. Behav. Neurosci. 1, 201–230 (2009)
Bénard, G. et al. Mitochondrial CB1 receptors regulate neuronal energy metabolism. Nat. Neurosci. 15, 558–564 (2012)
Hebert-Chatelain, E. et al. Cannabinoid control of brain bioenergetics: Exploring the subcellular localization of the CB1 receptor. Mol. Metab. 3, 495–504 (2014)
Koch, M. et al. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature 519, 45–50 (2015)
Claros, M. G. MitoProt, a Macintosh application for studying mitochondrial proteins. Comput. Appl. Biosci. 11, 441–447 (1995)
Nakai, K. & Horton, P. PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem. Sci. 24, 34–36 (1999)
Boesmans, W., Ameloot, K., van den Abbeel, V., Tack, J. & Vanden Berghe, P. Cannabinoid receptor 1 signalling dampens activity and mitochondrial transport in networks of enteric neurones. Neurogastroenterol. and Motil. 21, 958–968 (2009)
Kano, M., Ohno-Shosaku, T., Hashimotodani, Y., Uchigashima, M. & Watanabe, M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 89, 309–380 (2009)
Puighermanal, E. et al. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat. Neurosci. 12, 1152–1158 (2009)
Benincá, C. et al. A new non-canonical pathway of Gαq protein regulating mitochondrial dynamics and bioenergetics. Cell. Signal. 26, 1135–1146 (2014)
Lyssand, J. S. & Bajjalieh, S. M. The heterotrimeric G protein subunit Gαi is present on mitochondria. FEBS Lett. 581, 5765–5768 (2007)
Acin-Perez, R. et al. Modulation of mitochondrial protein phosphorylation by soluble adenylyl cyclase ameliorates cytochrome oxidase defects. EMBO Mol. Med. 1, 392–406 (2009)
Chen, Y. et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289, 625–628 (2000)
Pierre, S., Eschenhagen, T., Geisslinger, G. & Scholich, K. Capturing adenylyl cyclases as potential drug targets. Nat. Rev. Drug Discov. 8, 321–335 (2009)
Hess, K. C. et al. The ‘soluble’ adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev. Cell 9, 249–259 (2005)
Acin-Perez, R. et al. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 9, 265–276 (2009)
Livigni, A. et al. Mitochondrial AKAP121 links cAMP and src signaling to oxidative metabolism. Mol. Biol. Cell 17, 263–271 (2006)
Chen, Q., Lin, R. Y. & Rubin, C. S. Organelle-specific targeting of protein kinase AII (PKAII). Molecular and in situ characterization of murine A kinase anchor proteins that recruit regulatory subunits of PKAII to the cytoplasmic surface of mitochondria. J. Biol. Chem. 272, 15247–15257 (1997)
Niswender, C. M. et al. Cre recombinase-dependent expression of a constitutively active mutant allele of the catalytic subunit of protein kinase A. Genesis 43, 109–119 (2005)
Amanchy, R. et al. A curated compendium of phosphorylation motifs. Nat. Biotechnol. 25, 285–286 (2007)
Neuberger, G., Schneider, G. & Eisenhaber, F. pkaPS: prediction of protein kinase A phosphorylation sites with the simplified kinase-substrate binding model. Biol. Direct 2, 1–23 (2007)
Lin, A. L. et al. Decreased in vitro mitochondrial function is associated with enhanced brain metabolism, blood flow, and memory in Surf1-deficient mice. J. Cereb. Blood Flow Metab. 33, 1605–1611 (2013)
Roubertoux, P. L. et al. Mitochondrial DNA modifies cognition in interaction with the nuclear genome and age in mice. Nat. Genet. 35, 65–69 (2003)
Bockaert, J. & Marin, P. mTOR in brain physiology and pathologies. Physiol. Rev. 95, 1157–1187 (2015)
Pacher, P., Bátkai, S. & Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 58, 389–462 (2006)
Marsicano, G. et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature 418, 530–534 (2002)
Martín-Couce, L. et al. Chemical probes for the recognition of cannabinoid receptors in native systems. Angew. Chem. Int. Ed. Engl. 51, 6896–6899 (2012)
Narayana, N., Cox, S., Shaltiel, S., Taylor, S. S. & Xuong, N. Crystal structure of a polyhistidine-tagged recombinant catalytic subunit of cAMP-dependent protein kinase complexed with the peptide inhibitor PKI(5-24) and adenosine. Biochemistry 36, 4438–4448 (1997)
McClure, C., Cole, K. L., Wulff, P., Klugmann, M. & Murray, A. J. Production and titering of recombinant adeno-associated viral vectors. J. Vis. Exp. 27, e3348 (2011)
Soria-Gómez, E. et al. The endocannabinoid system controls food intake via olfactory processes. Nat. Neurosci. 17, 407–415 (2014)
Chiarlone, A. et al. A restricted population of CB1 cannabinoid receptors with neuroprotective activity. Proc. Natl Acad. Sci. USA 111, 8257–8262 (2014)
López-Doménech, G. et al. The Eutherian Armcx genes regulate mitochondrial trafficking in neurons and interact with Miro and Trak2. Nat. Commun. 3, 814 (2012)
Jose, C. et al. AICAR inhibits cancer cell growth and triggers cell-type distinct effects on OXPHOS biogenesis, oxidative stress and Akt activation. Biochim. Biophys. Acta 1807, 707–718 (2011)
Hébert Chatelain, E., Dupuy, J. W., Letellier, T. & Dachary-Prigent, J. Functional impact of PTP1B-mediated Src regulation on oxidative phosphorylation in rat brain mitochondria. Cell. Mol. Life Sci. 68, 2603–2613 (2011)
Pocaly, M. et al. Proteomic analysis of an imatinib-resistant K562 cell line highlights opposing roles of heat shock cognate 70 and heat shock 70 proteins in resistance. Proteomics 8, 2394–2406 (2008)
Käll, L., Canterbury, J. D., Weston, J., Noble, W. S. & MacCoss, M. J. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 4, 923–925 (2007)
Zala, D. et al. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell 152, 479–491 (2013)
De Vos, K. J. & Sheetz, M. P. Visualization and quantification of mitochondrial dynamics in living animal cells. Methods Cell Biol. 80, 627–682 (2007)
Courchet, J. et al. Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell 153, 1510–1525 (2013)
Rodríguez-Puertas, R., Barreda-Gómez, G., Giralt, M. & Fernández-Pastor, B. Method of quantifying the G protein-coupled receptor (GPCR)/G protein coupling using a cell membrane array. Patent US 8017346 B2. Google Patents (2013)
Rodriguez-Puertas, R. et al. Method for the surface treatment of solid substrates. Patent EP 2048534 A1 Google Patents (2009)
Busquets-Garcia, A. et al. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol. Psychiatry 70, 479–486 (2011)
Puighermanal, E. et al. Dissociation of the pharmacological effects of THC by mTOR blockade. Neuropsychopharmacology 38, 1334–1343 (2013)
Acknowledgements
We thank D. Gonzales,N. Aubailly and all the personnel of the Animal Facility of the NeuroCentre Magendie, M. Biguerie of the technical service of the NeuroCentre Magendie, the personnel from the Bordeaux Imaging Center and V. Morales for continuous help. We thank G. Manfredi (Cornell University) for the sAC–HA, A. Feliciello (University of Napoli) for anti-AKAP121 antiserum and for AKAP121 shRNA, and M. Montcouquiol, N. Piguel (INSERM U1215, Bordeaux) and R. Rossignol (University of Bordeaux) for help with experiments. We thank F. Francia, P. V. Piazza, A. Bacci, G. Ferreira, F. Chaouloff and M. Guzman for critical reading and the members of Marsicano’s laboratory for discussions. This work was supported by INSERM (G.M., D.C.), EU–Fp7 (PAINCAGE, HEALTH-603191, G.M. and FP7-PEOPLE-2013-IEF-623638, A.B.-G.), European Research Council (Endofood, ERC–2010–StG–260515 and CannaPreg, ERC-2014-PoC-640923, G.M.), Fondation pour la Recherche Medicale (DRM20101220445, G.M., SPF20121226369, R.S. and ARF20140129235, L.B.), Fondation pour la Recherche en Psychiatrie et en Santé Mentale (FRPSM, G.M.), Human Frontiers Science Program (RGP0036/2014, G.M.), Region Aquitaine (G.M.), AFM Telethon Trampoline Grant (16474, G.B.), Agence Nationale de la Recherche (ANR Blanc NeuroNutriSens ANR-13-BSV4-0006, G.M., D.C., BRAIN ANR-10-LABX-0043, G.M., D.C., F.M. and ANR-10-IDEX-03-02, A.B.-G.), Dulbecco Telethon Institute post-doc fellowship (E.H.-C.), NSERC (RGPIN-2015-05880, E.H.-C.), Fyssen Foundation (E.S.-G.), EMBO post-doc fellowship (L.B.), CONACyT (E.S.-G.), Zabalduz pre-doc fellowship (M.D.G.-F.), the Basque Government (IT764-13, P.G.), MINECO/FEDER (SAF2015-65034-R, P.G.), University of the Basque Country (UPV/EHU UFI11/41, P.G.), Red de Trastornos Adictivos—Instituto de Salud Carlos III (RD12/0028/0004, RD16/0017/0012, P.G.).
Author information
Authors and Affiliations
Contributions
E.H.-C., T.D., R.S. and L.B. performed biochemical, molecular biology, behavioural and cellular experiments; E.S.-G., A.B.-G. and L.M.R. helped with behavioural experiments and analyses; E.S.-G., A.C.P.Z., A.C., A.D., P.V. and M.V. helped with biochemistry and molecular biology; G.T., M.C., F.D., W.M., D.C. and F.M. performed electrophysiological studies; M.D.G.-F. and G.B.-G. performed G protein signalling and binding experiments; N.P., L.R., I.E. and P.G. performed electron microscopy experiments; J.-W.D. provided proteomics experiments; M.-L.L.-R. provided reagents; G.B. and G.M. supervised the work; E.H.-C., T.D., G.B. and G.M. wrote the manuscript; all authors discussed results and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information
Nature thanks M. Mattson and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 The mutant DN22-CB1 receptor is functional, but it does not mediate cannabinoid-induced alterations of mitochondrial activity and mobility.
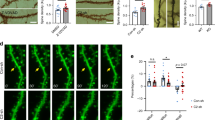
a, Acute treatment with HU210 (100 nM) induces changes in cellular oxygen consumption in intact primary fibroblasts (MFs) from CB1−/− mice electroporated with a CB1-expressing plasmid, whereas no alterations were observed in DN22-CB1-expressing or mock-electroporated (mCherry) CB1−/− mouse fibroblasts (n = 6–10). b, HU210 (200 nM) treatment decreases HEK293 cellular respiration in cells expressing CB1, but not mCherry or DN22-CB1 receptors (n = 4). c, Representative immunoblotting showing the effect of HU210 (100 nM) on pERK and ERK levels in HEK293 cells transfected with plasmids expressing mCherry, CB1 or DN22-CB1 (n = 7–13). Quantitative data are shown in Fig. 1b of main text. d, Representative confocal images (green, GFP; red, pDsred2–mito) and kymographs corresponding to the results shown in Fig. 1d; showing how HU210 (1 μM) reduces mitochondrial mobility in the primary neurons from CB1−/− mice transfected with vectors expressing CB1, but not GFP or DN22-CB1. Scale bar, 10 μM. Data, mean ± s.e.m.; *P < 0.05. For statistics, see Supplementary Tables 1–3.
Extended Data Figure 2 Characterization of CB1+/+(GFP), CB1−/−(GFP), CB1−/−(CB1) and CB1−/−(DN22-CB1) mice.
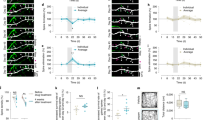
a, Representative immunofluorescence micrographs showing CB1-receptor expression in hippocampal brain sections from CB1+/+(GFP), CB1−/−(GFP), CB1−/−(CB1) and CB1−/−(DN22-CB1) mice, respectively. b, [3H]CP55,940 CB1-receptor binding experiments, showing similar levels of CB1 protein expression in hippocampal extracts from CB1−/−(CB1) and CB1−/−(DN22-CB1) mice, both significantly higher than control CB1−/−(GFP) (n = 7–24). c, Dose–response curves of [35S]GTPγS binding in membranes isolated from hippocampi of CB1−/−(GFP), CB1−/−(CB1) and CB1−/−(DN22-CB1) mice treated with WIN (n = 9–24). d, e, CB1-receptor-dependent activation of G proteins is similar in hippocampi from CB1−/−(CB1) and CB1−/−(DN22-CB1) mice, as shown by EC50 dose and maximal activation values calculated from [35S]GTPγS binding experiments after treatment with HU210 (d) or WIN (e). f, Total exploration time in NOR experiments as described in Fig. 1i. Data, mean ± s.e.m.; ***P < 0.001; NS, not significant. For statistics, see Supplementary Tables 1–3.
Extended Data Figure 3 Activation of mtCB1 receptor affects mitochondrial activity via Gαi/o signalling.
a, THC (1 μM) or WIN (1 μM) decrease cellular respiration of primary hippocampal cultures derived from CB1+/+ but not from CB1−/− mice (n = 5–15). b, THC (1 μM) or WIN (1 μM) reduce both total and mitochondrial ATP levels of primary hippocampal cultures derived from CB1+/+ but not from CB1−/− mice (n = 6–14). c, Quantification of data presented in Fig. 2b, showing the release of the G protein upon activation of mtCB1 receptors (n = 3). d, e, Effects of pertussis toxin (PTX, 1 μg ml−1) and THC (800 nM) on cAMP levels in purified brain mitochondria obtained from CB1+/+ mice (d) and CB1−/− littermates (e) (n = 4). f, g, Effects of PTX (1 μg ml−1) and THC (800 nM) on PKA activity in purified brain mitochondria obtained from CB1+/+ mice (f) and CB1−/− littermates (g) (n = 5–6). h, PTX (1 μg ml−1) blocks the effect of THC (800 nM) and WIN (100 nM) on complex-I activity of purified brain mitochondria obtained from CB1+/+ mice (n = 3–6). i, Representative trypsin-sensitivity assay (3 independent experiments) showing the intra-mitochondrial localization of Gα, sAC and PKA. NDUFA9, a subunit of complex I; TOM20, translocase of outer membrane subunit 20. Data, mean ± s.e.m.; *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant. For statistics, see Supplementary Tables 1–3.
Extended Data Figure 4 sAC mediates the mitochondrial effects of mtCB1-receptor activation.
a, Quantification of data presented in Fig. 2e, indicating higher interaction between Gα proteins and sAC after mtCB1 activation (n = 3). b, The transmembrane adenylyl cyclase activator forskolin (10 μM) does not alter basal levels and does not reverse the effect of THC (800 nM) on cAMP content of CB1+/+ brain mitochondria (n = 4). c, Forskolin (10 μM) has no effect on basal cAMP levels of CB1−/− brain mitochondria (n = 3–4). d, The sAC activator HCO3− (5 mM) reverses the effect of THC (800 nM) on cAMP content in purified brain mitochondria from CB1+/+ mice (n = 4). e, HCO3− (5 mM) and THC (800 nM) have no effect on cAMP levels (n = 3–4) of CB1−/− brain mitochondria. f, Representative trypsin sensitivity assay in mitochondrial fractions of 3T3 cells (3 independent experiments) showing intra-mitochondrial localization of CB1 receptors, Gα proteins, AKAP121, PKA and sAC in 3T3 cells. SDHA, succinate dehydrogenase A (subunit of complex II); TOM20, translocase of outer membrane subunit 20. g, Representative trypsin-sensitivity assay (3 independent experiments) showing that sAC–HA and mtsAC–HA are localized inside mitochondria in transfected 3T3 cells. h, Representative immunoblot (3 independent experiments) showing the expression of sAC–HA in transfected and non-transfected primary hippocampal cultures. i, Expression of sAC–HA in primary hippocampal cultures blocks the effect of THC (1 μM) on basal cellular respiration (n = 8–10). Data, mean ± s.e.m.; P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant. For statistics, see Supplementary Tables 1–3.
Extended Data Figure 5 The sAC inhibitor KH7 prevents the effects of mtCB1 activation.
a, The effect of KH7 (5 μM) on mitochondrial respiration in purified brain mitochondria obtained from CB1+/+ mice (n = 5–6). b, KH7 (5 μM) blocks the effect of THC (800 nM) on PKA activity in purified brain mitochondria obtained from CB1+/+ mice (n = 5–11). c, KH7 (5 μM) blocks the effect of THC (800 nM) and WIN (100 nM) on complex-I activity in purified brain mitochondria obtained from CB1+/+ mice (n = 3–6). d, Representative confocal images (green, CB1-GFP; red, pDsred2–mito) of analysed axonal tracts and kymographs of the results shown in Fig. 3b, representing the effect of KH7 (5 μM) on the reduction in mitochondrial mobility in primary hippocampal neurons induced by HU210 (1 μM). Scale bar, 10 μM. e, mtsAC–HA blocks the decrease in mitochondrial mobility in CB1-expressing neurons induced by HU210 (1 μM) (n = 7–18). f, The effect of KH7 (10 μM) on the decrease in fEPSP at CA3–CA1 synapses from C57BL/6N mice induced by WIN (5 μM). Left, plots of normalized fEPSP slopes in vitro with representative fEPSP traces before (1) and after (2) WIN incubation. Vehicle (n = 6) or KH7 (n = 5) were pre-incubated 10 min before cannabinoid application. Right, histogram summarizing the average changes in the percentage of the fEPSP slope before (100% baseline, dotted line) and after WIN treatment in the presence or absence of KH7. g, KH7 (10 μM) alone does not affect the fEPSP slope at CA3–CA1 synapses from C57BL/6N mice. Plots of normalized fEPSP slopes in vitro before and after KH7 application (n = 5). h, Total exploration time in NOR experiments as described in Fig. 3d. Data, mean ± s.e.m.; *P < 0.05; ***P < 0.001; NS, not significant. For statistics, see Supplementary Tables 1–3.
Extended Data Figure 6 Mitochondrial PKA activity mediates the effects of mtCB1 receptors.
a, The PKA inhibitor H89 (800 nM) blocks the effect of THC (800 nM) and WIN (100 nM) on complex-I activity in CB1+/+ purified brain mitochondria (n = 3–6). b, H89 blocks the effect of THC on PKA activity in CB1+/+ purified brain mitochondria (n = 6). c, H89 and THC have no effect on PKA activity in CB1−/− brain mitochondria (n = 3–5). d, Quantification of data presented in Fig. 4c (n = 3), indicating a lower mitochondrial PKA content after silencing of AKAP121. e, f, Silencing of AKAP121 lowers mitochondrial PKA activity. e, Representative immunoblots of proteins phosphorylated by PKA (PKA-dependent phosphorylation) in 3T3 cells transfected with control vector or shAKAKP121. SHDA, succinate dehydrogenase A (a subunit of complex II). f, Quantification of data presented in e (n = 12). g–i, Silencing of AKAP121 blocks the effect of THC on cellular respiration. g, Silencing of AKAP121 blocks the effect of THC (800 nM) on basal cellular respiration in 3T3 cells (n = 4). h, Representative immunoblotting of AKAP121 expression in extracts of hippocampal primary cultures in the presence or absence of shAKAP121. i, shAKAP121 inhibits the effect of THC on basal cellular respiration of primary hippocampal cultures (n = 5–10). Data, mean ± s.e.m.; *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant. For statistics, see Supplementary Tables 1–3.
Extended Data Figure 7 Activation of mtCB1 decreases PKA-dependent phosphorylation of the complex-I subunit NDUFS2, eventually mediating the effects on respiration.
a, THC specifically decreases complex-I- but not complex-II- or complex-IV-dependent respiration in purified brain mitochondria in a CB1-dependent manner (n = 3–4). b, Representative two-dimension (BN-PAGE/SDS–PAGE) immunoblotting (4 independent experiments) showing PKA-dependent phosphorylation of protein extracts of brain mitochondria from CB1+/+ and CB1−/− mice treated with vehicle or THC (800 nM). c, Representative immunofluorescence of HeLa cells transfected with empty vector (mock), the mitochondrial-targeted constitutively active PKA (MLS–PKA-CA) or the phosphomimetic mutant of NDUFS2 (NDUFS2-PM). Green, myc tag staining; red, mitochondrial staining (TOM20). d, Baseline cellular respiration of HEK293 cells transfected with plasmids expressing CB1, CB1 + MLS–PKA-CA or CB1 + NDUFS2-PM (n = 4–6). e, The effect of vehicle or WIN on cellular respiration in HEK293 cells as in d (n = 4–6). f, Representative images of hippocampi injected with AAV–GFP, AAV–MLS–PKA-CA or AAV–NDUFS2-PM. Green, GFP fluorescence (left panel) or myc tag staining (central and right panel). Arrows, subunit of complex I, NDUFS2. ***P < 0.001 between genotypes (a) or between vehicle and WIN (d); ##P < 0.01; ###P < 0.001 as compared to control. For statistics details, see Supplementary Tables 1–3.
Supplementary information
Supplementary Information
This file contains Supplementary Figure 1, which shows uncropped Western Immunoblotting images used for the relative panels of main figures. (PDF 1222 kb)
Cannabinoids reduce mitochondrial mobility in hippocampal neurons via CB1 receptors
Effect of the CB1 receptor agonist HU210 on mitochondrial mobility in CB1-/- hippocampal neurons re-expressing CB1. Representative time-lapse live imaging of axonal mitochondria before and after treatment of HU210 (1µM) for 15 min in a primary hippocampal neuron from CB1-/- mice, transfected with CB1-GFP. See Fig. 1e. (MOV 643 kb)
Cannabinoid-induced reduction of mitochondrial mobility depends on mtCB1 receptors
Re-expression of DN22-CB1 fails to rescue the HU210-dependent decrease of mitochondrial mobility in CB1-/- hippocampal neurons. Representative time-lapse live imaging of axonal mitochondria before and after treatment of HU210 (1µM) for 15 min in a primary hippocampal neuron from CB1-/- mice, transfected with DN22-CB1-GFP. See Fig. 1e. (MOV 700 kb)
Cannabinoid-induced reduction of mitochondrial mobility depends on sAC signaling.
Representative time-lapse live imaging of axonal mitochondria before and after treatment of HU210 (1µM) and vehicle for 15 min in a primary hippocampal neuron from CB1-/- mice, transfected with CB1-GFP. See Fig. 3b. (MOV 731 kb)
Cannabinoid-induced reduction of mitochondrial mobility depends on sAC signaling.
The sAC inhibitor KH7 blocks the reduction of mitochondrial mobility induced by HU210. Representative time-lapse live imaging of axonal mitochondria before and after treatment of HU210 (1µM) for 15min in the presence of KH7 (5µM) in a primary hippocampal neuron from CB1-/- mice, transfected with CB1-GFP. See Fig. 3b. (MOV 771 kb)
Source data
Rights and permissions
About this article
Cite this article
Hebert-Chatelain, E., Desprez, T., Serrat, R. et al. A cannabinoid link between mitochondria and memory. Nature 539, 555–559 (2016). https://doi.org/10.1038/nature20127
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature20127
This article is cited by
-
Research progress in the management of vascular disease with cannabidiol: a review
Journal of Cardiothoracic Surgery (2024)
-
The synthetic cannabinoids menace: a review of health risks and toxicity
European Journal of Medical Research (2024)
-
Anandamide and WIN 55212–2 Afford Protection in Rat Brain Mitochondria in a Toxic Model Induced by 3-Nitropropionic Acid: an In Vitro Study
Molecular Neurobiology (2024)
-
Carbon dioxide and MAPK signalling: towards therapy for inflammation
Cell Communication and Signaling (2023)
-
Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction
Nature Metabolism (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.