Abstract
Spinal cord injury disrupts the communication between the brain and the spinal circuits that orchestrate movement. To bypass the lesion, brain–computer interfaces1,2,3 have directly linked cortical activity to electrical stimulation of muscles, and have thus restored grasping abilities after hand paralysis1,4. Theoretically, this strategy could also restore control over leg muscle activity for walking5. However, replicating the complex sequence of individual muscle activation patterns underlying natural and adaptive locomotor movements poses formidable conceptual and technological challenges6,7. Recently, it was shown in rats that epidural electrical stimulation of the lumbar spinal cord can reproduce the natural activation of synergistic muscle groups producing locomotion8,9,10. Here we interface leg motor cortex activity with epidural electrical stimulation protocols to establish a brain–spine interface that alleviated gait deficits after a spinal cord injury in non-human primates. Rhesus monkeys (Macaca mulatta) were implanted with an intracortical microelectrode array in the leg area of the motor cortex and with a spinal cord stimulation system composed of a spatially selective epidural implant and a pulse generator with real-time triggering capabilities. We designed and implemented wireless control systems that linked online neural decoding of extension and flexion motor states with stimulation protocols promoting these movements. These systems allowed the monkeys to behave freely without any restrictions or constraining tethered electronics. After validation of the brain–spine interface in intact (uninjured) monkeys, we performed a unilateral corticospinal tract lesion at the thoracic level. As early as six days post-injury and without prior training of the monkeys, the brain–spine interface restored weight-bearing locomotion of the paralysed leg on a treadmill and overground. The implantable components integrated in the brain–spine interface have all been approved for investigational applications in similar human research, suggesting a practical translational pathway for proof-of-concept studies in people with spinal cord injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ethier, C., Oby, E. R., Bauman, M. J. & Miller, L. E. Restoration of grasp following paralysis through brain-controlled stimulation of muscles. Nature 485, 368–371 (2012)
Collinger, J. L. et al. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet 381, 557–564 (2013)
Hochberg, L. R. et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 485, 372–375 (2012)
Bouton, C. E. et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature 533, 247–250 (2016)
Mushahwar, V. K., Guevremont, L. & Saigal, R. Could cortical signals control intraspinal stimulators? A theoretical evaluation. IEEE Trans. Neural Syst. Rehabil. Eng. 14, 198–201 (2006)
Ho, C. H. et al. Functional electrical stimulation and spinal cord injury. Phys. Med. Rehabilitation Clinics North Am. 25, 631–654 (2014)
Kapadia, N. et al. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: effects on walking competency. J. Spinal Cord Med. 37, 511–524 (2014)
Wenger, N. et al. Closed-loop neuromodulation of spinal sensorimotor circuits controls refined locomotion after complete spinal cord injury. Sci. Transl. Med. 6, 255ra133 (2014)
Wenger, N. et al. Spatiotemporal neuromodulation therapies engaging muscle synergies improve motor control after spinal cord injury. Nat. Med. 22, 138–145 (2016)
Moraud, E. M. et al. Mechanisms underlying the neuromodulation of spinal circuits for correcting gait and balance deficits after spinal cord injury. Neuron 89, 814–828 (2016)
Sherrington, C. S. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J. Physiol. 40, 28–121 (1910)
Kiehn, O. Decoding the organization of spinal circuits that control locomotion. Nat. Rev. Neurosci. 17, 224–238 (2016)
Holinski, B. J., Everaert, D. G., Mushahwar, V. K. & Stein, R. B. Real-time control of walking using recordings from dorsal root ganglia. J. Neural Eng. 10, 056008 (2013)
Angeli, C. A., Edgerton, V. R., Gerasimenko, Y. P. & Harkema, S. J. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 137, 1394–1409 (2014)
Gerasimenko, Y. P. et al. Noninvasive reactivation of motor descending control after paralysis. J. Neurotrauma 32, 1968–1980 (2015)
Danner, S. M. et al. Human spinal locomotor control is based on flexibly organized burst generators. Brain 138, 577–588 (2015)
Barthelemy, D., Leblond, H. & Rossignol, S. Characteristics and mechanisms of locomotion induced by intraspinal microstimulation and dorsal root stimulation in spinal cats. J. Neurophysiol. 97, 1986–2000 (2007)
Shenoy, K. V. & Carmena, J. M. Combining decoder design and neural adaptation in brain–machine interfaces. Neuron 84, 665–680 (2014)
Shanechi, M. M., Hu, R. C. & Williams, Z. M. A cortical-spinal prosthesis for targeted limb movement in paralysed primate avatars. Nat. Commun. 5, 3237 (2014)
Zimmermann, J. B. & Jackson, A. Closed-loop control of spinal cord stimulation to restore hand function after paralysis. Front. Neurosci. 8, 87 (2014)
Nishimura, Y., Perlmutter, S. I., Eaton, R. W. & Fetz, E. E. Spike-timing-dependent plasticity in primate corticospinal connections induced during free behavior. Neuron 80, 1301–1309 (2013)
Lemon, R. N. Descending pathways in motor control. Annu. Rev. Neurosci. 31, 195–218 (2008)
Friedli, L. et al. Pronounced species divergence in corticospinal tract reorganization and functional recovery after lateralized spinal cord injury favors primates. Sci. Transl. Med. 7, 302ra134 (2015)
Courtine, G. et al. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat. Med. 13, 561–566 (2007)
Yin, M. et al. Wireless neurosensor for full-spectrum electrophysiology recordings during free behavior. Neuron 84, 1170–1182 (2014)
Yakovenko, S., Mushahwar, V., VanderHorst, V., Holstege, G. & Prochazka, A. Spatiotemporal activation of lumbosacral motoneurons in the locomotor step cycle. J. Neurophysiol. 87, 1542–1553 (2002)
Rattay, F., Minassian, K. & Dimitrijevic, M. R. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 2. Quantitative analysis by computer modeling. Spinal Cord 38, 473–489 (2000)
Courtine, G. & Bloch, J. Defining ecological strategies in neuroprosthetics. Neuron 86, 29–33 (2015)
Shoham, S., Halgren, E., Maynard, E. M. & Normann, R. A. Motor-cortical activity in tetraplegics. Nature 413, 793 (2001)
van den Brand, R. et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 336, 1182–1185 (2012)
Courtine, G. et al. Kinematic and EMG determinants in quadrupedal locomotion of a non-human primate (Rhesus). J. Neurophysiol. 93, 3127–3145 (2005)
Fraser, G. W., Chase, S. M., Whitford, A. & Schwartz, A. B. Control of a brain-computer interface without spike sorting. J. Neural Eng. 6, 055004 (2009)
Gilja, V. et al. Clinical translation of a high-performance neural prosthesis. Nat. Med. 21, 1142–1145 (2015)
Aflalo, T. et al. Decoding motor imagery from the posterior parietal cortex of a tetraplegic human. Science 348, 906–910 (2015)
Milekovic, T., Ball, T., Schulze-Bonhage, A., Aertsen, A. & Mehring, C. Detection of error related neuronal responses recorded by electrocorticography in humans during continuous movements. PLoS One 8, e55235 (2013)
Marieb, E. N. Human Anatomy and Physiology 6th edn (Pearson Education, 2003)
Kuypers, H. G. J. M. in Comprehensive Physiology Ch. 3 (John Wiley & Sons, 2011)
Acknowledgements
G.C. holds the International Foundation for Research in Paraplegia Chair in Spinal Cord Repair. S.M. holds the Bertarelli Foundation Chair in Translational Neuroengineering. We thank X. Rulin and C. Yunlong for providing support, taking care of the monkeys, performing behavioural training, and collecting data; E. Pirondini, N. Pavlova and P. Musienko for help with experiments; J. Courtine, I. Pitteloud, J. Rubattel, L. Dalang and R. Hasler for help with kinematic reconstruction; J. Courtine for the voice-over in video; J. Kreider for help with anatomy; and J. Laurens for discussions and photographs. The illustrations were created by Jemère Ruby. This work was supported by Medtronic, the European Community's Seventh Framework Program (CP-IP 258654, NeuWALK), the International Paraplegic foundation, a Starting Grant from the European Research Council (ERC 261247, Walk Again), the Wyss centre in Geneva, a Marie Curie Fellowship to D.B. (331602, e-WALK), Marie Curie COFUND EPFL fellowships to T.M. and F.W., a Morton Cure Paralysis Fund fellowship to T.M., and the Swiss National Science Foundation including the National Centre of Competence in Research in Robotics, the Sinergia program (CRSII3_160696), the Sino-Swiss Science and Technology Cooperation (IZLCZ3_156331), and the NanoTera.ch programme (SpineRepair).
Author information
Authors and Affiliations
Contributions
M.C., T.M. and D.B. contributed equally to this work. F.W. and E.M.M. contributed equally to this work. S.M., E.B. and J.B. contributed equally to this work. M.C. developed the spinal cord stimulation protocols and the routines for the identification of flexion and extension hotspots. T.M. developed the brain decoder and the decoder calibration routines. D.B. developed the experimental platform. M.C., T.M., F.W. and E.M.M. performed all the behavioural experiments (with help from D.B., J.G., Y.J. and G.C.). M.C., T.M. and F.W. analysed the data (with input from E.M.M., J.-B.M. and D.X.). M.C., T.M., F.W., E.M.M. and J.G. developed the real-time software application. N.B. and T.D. developed the Neural Research Programmer (with input from M.C., D.B., T.M., F.W. and J.G.). Q.B. and E.R. processed the anatomical data. Y.J. trained all the monkeys. W.K.D.K., Q.L. and E.B. managed the experimental protocols and procedures. P.D. developed and produced the spinal implants (from designs by M.C., D.B., J.B. and G.C.). J.B., D.B., Q.L. and G.C. performed the surgeries. G.C., S.M., E.B., J.B. and P.D. secured funding for the study. G.C. conceived and supervised the study. G.C. wrote the paper with M.C., T.M. and F.W., and all the authors contributed to its editing.
Corresponding author
Ethics declarations
Competing interests
G.C., D.B., M.C., S.M., E.M.M. and J.B. hold various patents related to the present work. T.D. and N.B. are Medtronic employees and contributed to the technical accuracy of the work but did not influence the results or the content of the manuscript. E.B. reports receipt of personal fees from Motac Neuroscience Ltd UK and is a shareholder of Motac Holding UK and Plenitudes SARL France. G.C., S.M. and J.B. are founders and shareholders of G-Therapeutics BV.
Additional information
Reviewer Information
Nature thanks A. Jackson, A. Prochazka, S. Scott and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
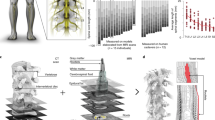
Extended Data Figure 1 Design and fabrication of the spatially selective spinal implant.
Step 1: Quantification of the length of vertebrae T12 to L4, and of spinal segments L1 to S1. The photographs show coronal sections of the left halves of the L3 and S1 spinal segments, including the dura mater, dorsal roots and spinal tissue. The complementary views show camera lucida reconstructions of the dorsal roots, white and grey matter. The dorsal roots projecting to the L1/L2 and L6/L7 spinal segments are colour-coded to visualize their respective location. Step 2: Three-dimensional reconstruction of the entire lumbosacral spinal cord and dorsal roots. The dorsal roots innervating spinal segments containing the targeted extension (L6, L7) and flexion (L1, L2) hotspots are colour-coded to help visualize their respective spatial trajectory. The three-dimensional reconstructions are displayed from a dorsal view, and from a view that is rotated 45° leftward around the rostrocaudal axis to visualize the trajectory of the dorsal roots along spinal segments. Step 3: Design of the spatially selective spinal implants, including the location of the electrodes with respect to the dorsal roots, and positioning of the implants with respect to the vertebrae. The cyan and magenta shapes highlight the electrodes targeting the flexor and extensor hotspots, respectively. Step 4: Mask layout of the spatially selective epidural spinal implant. Step 5: Fabrication of the spinal implants. Processing starts with deposition of a 40-μm-thick polyimide film by spin coating on the silicon substrate. The polyimide-based bottom layer constituting the footprint of the implant is realized through ultraviolet lithography. A 200-nm-thick gold layer is deposited using vacuum evaporation and lithographically structured to create a conductive seed pattern. An additional gold layer is electroplated to a height of approximately 6 μm to create the electrodes and interconnects. A 20-μm-thick cover layer of photosensitive polyimide is then applied by spin coating to uniformly cover the bottom and electrode structure. A final ultraviolet lithography is applied to structure the top layer of the implant, and to create openings over the electrodes and contact pads. The implants are gently released from the carrier wafer. To create a connector, the contact pads located at the extremity of the implant are glued to a small support plate made of medical-grade poly-methyl methacrylate (PMMA) sheet material. Precision milled alignment structures allow fine adjustment of the support plate to the spinal implant, and to the extremity of the leads. The support plate contains a seat in which a tiny printed circuit board with conductive stripes is fixed. Stainless steel leads with silicone rubber insulation are directly soldered to the contact pads of the implant via the contact stripes located on the printed circuit board. The resulting implants and connective leads were highly reliable mechanically and electrically. A fixation structure was realized by using three-dimensional laser sintering of medical grade titanium. Step 6: Photographs, including zoomed insets, showing a fabricated spinal implant and a micro-computed tomography scan performed after implantation. Photographs, copyright J. Laurens (2016).
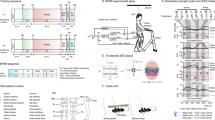
Extended Data Figure 2 Protocols and technology of the spinal cord stimulation system.
Step 1: A Neural Research Programmer interface (screen snapshot) encodes stimulation protocols that are pre-programmed into a table uploaded to the implantable pulse generator. Each row of this table corresponds to a specific electrode configuration (cathodes and anodes) and stimulation features (amplitude, frequency, pulse width and duration of stimulation). During experiments, the control computer selects the rows to be executed. The plot reports the distribution of temporal delays introduced by the communication between the decoder and the Neural Research Programmer (n = 5,000). Step 2: Stimulation commands are transmitted to the implantable pulse generator. Commands are first broadcast via Bluetooth to a module that converts them into infrared signals transferred to the stimulation programmer device. The Bluetooth-to-infrared module and the stimulation programmer were embedded into a jacket worn by the monkeys during the experiments. The stimulation programmer transmitted the stimulation commands into the implantable pulse generator via induction telemetry. The antenna was placed under the jacket, in contact with the skin and aligned to the implantable pulse generator. The plot reports the distribution of delays needed to transmit the stimulation commands from the Neural Research Programmer to the implantable pulse generator. Step 3: The implantable pulse generator executed the selected stimulation protocols. After execution of the stimulation command, the implantable pulse generator switched to idle mode. The shape of a single charge balanced cathodic pulse is shown in the inset. The plot reports the distribution of time delays required to execute a single stimulation command by the implantable pulse generator. Photographs, copyright Medtronic (2016); drawing, copyright Jemère Ruby (2016).
Extended Data Figure 3 Anatomical, computational and functional experiments allowed the identification of stimulation protocols to access flexion and extension hotspots.
a, Computational procedure to estimate spatiotemporal maps of motoneuron activation during locomotion. Step 1: Four pairs of antagonist muscles spanning each joint of the leg are implanted with bipolar electrodes to record electromyographic signals during locomotion. Step 2: Muscle activity recorded during locomotion on a treadmill is band-pass-filtered using a Butterworth 3rd-order filter (30–800 Hz, monkey P3). Step 3: The signals are rectified, filtered with a low pass at 10 Hz, normalized to the maximum activity recorded across all the gait cycles, and then projected onto the location of the corresponding motoneuron columns in the spinal cord for each of the recorded muscles. The estimated motoneuron activation is represented as a colour-coded spatiotemporal map of motoneuron activation. b, Spatiotemporal maps of motoneuron activation recorded in three intact monkeys (Q1, P2 and P3). The maps were obtained by averaging electromyographic signals recorded during continuous locomotion on a treadmill (n = 73, 25 and 24 steps for monkeys Q1, P2 and P3, respectively). The maps underlying the activation of extension and flexion hotspots were extracted by averaging the estimated motoneuron activation around the foot-strike and foot-off events, respectively. For this, a window was defined from −10% to +30% of the gait cycle duration for the foot-strike event, and from −10% to +20% of the gait cycle duration for the foot-off event. The maps were reproducible across monkeys. The correlation between monkeys Q1, P2 and P3 for the flexion hotspot was 0.94, 0.90 and 0.90 for Q1–P2, Q1–P3 and P2–P3, respectively. The correlation between monkeys Q1, P2 and P3 for the extension hotspot was 0.88, 0.90 and 0.60 for Q1–P2, Q1–P3 and P2–P3, respectively. The resulting maps were projected onto the reconstructed spinal segments (Extended Data Fig. 1). c, Recruitment curves showing the relationships between motor-evoked potentials elicited by single pulses of epidural electrical stimulation in each of the recorded hindlimb muscles and the stimulation amplitude for three intact monkeys (Q1, Q2 and P1). Stimulation was delivered through the electrodes targeting the extension and flexion hotspots. The compound responses elicited in leg muscles were rectified and integrated to calculate the amplitude of the responses, and then projected on the reconstructed spinal segments. The spatial maps of motoneuron activation corresponding to the optimal range of stimulation amplitudes to stimulate the hotspots are displayed for each monkey, including the location of the electrodes with respect to spinal segments. To compute the optimal range of stimulation amplitudes for each hotspot, we extracted the stimulation amplitudes for which the spatial map of motoneuron activation displayed the highest values of correlation with the spatial maps of the targeted hotspots. The cyan and magenta shadings highlight the functional range of stimulation amplitude for each hotspot and monkey. (a.u., arbitrary units.)
Extended Data Figure 4 Procedure to calibrate the decoders for real-time detection of motor states.
Step 1: The locomotor movements of the right leg were recorded using the video camera system. In parallel, neural signals were recorded from the microelectrode array implanted into the leg area of the primary motor cortex. The signals were band-pass filtered (0.5–7.5 kHz). A threshold was at 3–3.5 times the standard deviation in order to obtain spike events. The two data streams were saved onto computers. Step 2: Visual inspection of the video frames allowed the identification of foot-off and foot-strike gait events. We estimated the spike rates from overlapping 150-ms bins that were updated every 20 ms. Gait events were then synchronized with the spike rate estimates using a trigger saved with the neural data that marked the onset of video recordings. Step 3: We extracted feature vectors that originated at foot-off and foot-strike events and assigned them to ‘foot-off’ and ‘foot-strike’ motor state classes, respectively. All other feature vectors were assigned to the ‘neither’ class. Step 4: Motor state classes of feature vectors were used to calibrate a regularized linear discriminant analysis decoder. Step 5: The decoder was uploaded into our real-time analysis application running on the control computer. Neural data was collected in real time, processed into spike-rate estimates, and passed through the decoder that calculated the probabilities of foot-off and foot-strike motor states. When one of the motor state probabilities crossed a threshold of 0.8, a command to trigger the flexion or extension hotspot stimulation protocols was sent to the Neural Research Programmer, which relayed this instruction to the implanted pulse generator. Owing to the wireless communication, the command was executed 178 ms (Q1) or 105 ms (Q2 and Q3) after the detection of the motor states.
Extended Data Figure 5 The real-time decoder accurately detected the motor states and triggered stimulation protocols during locomotion and when initiating and terminating it in intact monkeys.
a, Example of a continuous sequence of locomotion (20 s) during brain-controlled flexion and extension stimulation in an intact monkey (Q2, pre-lesion session 2; see Supplementary Table 2). Conventions are the same as in Fig. 3. The real-time decoder correctly detected the succession of 22 foot-off and foot-strike motor states occurring in this sequence (vertical dotted lines), and appropriately triggered the relevant stimulation protocols throughout the locomotor sequence. b, Histograms showing the distribution of the temporal differences between the actual occurrence of foot-off and foot-strike events and the decoded occurrence of these motor states for all the recording sessions of the tested intact monkeys (Q1 and Q2). The dotted lines indicate the median for each distribution. For monkey Q1, the decoders were calibrated using recordings without stimulation only. For monkey Q2, we improved the accuracy of the decoders by performing the calibration twice. First, the decoders were recalibrated using recordings without stimulation. These decoders were used to collect recordings during brain-controlled flexion or brain-controlled extension independently. New decoders were then calibrated using all the recording blocks together. c, Confusion matrices reporting the accuracy of the real-time decoders without stimulation and during brain-controlled stimulation, given a tolerance window of ±125 ms. The bar plots report the normalized mutual information calculated for the real-time decoders compared to random decoders and chance level decoders. Random decoders were the same decoders calibrated using shuffled gait events. The chance level decoders randomly assigned motor states with chance level probabilities, which were estimated from the data used to calibrate the online decoders, and at the same rate as the online decoders. ***P < 0.001, bootstrap. Error bars, s.e.m. d, Two examples of recordings collected while monkey Q1 and Q2 initiated or terminated sequences of continuous locomotion on a treadmill. The green shaded area indicates the period during which the treadmill was turned off. The brain–spine interface was kept on throughout the recordings. Conventions are the same as in Fig. 3. The probability of foot-strike and foot-off motor states remained low during the periods of rest, and recommenced to modulate with the occurrence of motor states when the monkey resumed continuous locomotion. The decoder confusion matrices were calculated during brain-controlled stimulation across all the sessions with intact monkeys during which the treadmill was turned off (n = 345 and n = 127 temporal windows for Q1 and Q2). False-positive detections were rare.
Extended Data Figure 6 Modulation of leg locomotor movements during brain-controlled stimulation in intact monkeys.
a, Principal component analysis was applied on individual gait cycles extracted from locomotion on a treadmill without stimulation (n = 125 and n = 119 for Q1 and Q2) and during brain-controlled stimulation of the extension (n = 33 and n = 54 gait cycles with stimulation for Q1 and Q2, respectively) or flexion (n = 98 and n = 120 total stimulation steps for Q1 and Q2, respectively) hotspots for the intact monkeys Q1 and Q2. Conventions are the same as in Fig. 3 and Extended Data Fig. 7. This analysis emphasizes the graded modulation of gait parameters when increasing the frequency or amplitude of stimulation for extension and flexion hotspots independently. b, Plots showing relationships between the amplitude or frequency of extensor (magenta) or flexor (cyan) hotspot stimulation and relevant kinematic or muscle activity parameter related to the extension or flexion of the right leg. The upper right plot shows the average foot trajectories during each experimental condition, illustrating the graded modulation of flexion during brain-controlled stimulation of the flexion hotspot. The lower right plot highlights the high degree of leg-specific modulation over the entire extent of tested stimulation parameters. Results were comparable in monkeys Q1 and Q2. *P < 0.05, **P < 0.001, Wilcoxon rank-sum test. Error bars, s.e.m.
Extended Data Figure 7 Quantification and reconstruction of the spinal cord lesions and quantification of gait deficits and spontaneous recovery after the spinal cord lesion.
a, Scheme illustrating the anatomical experiments to quantify the lesion of the corticospinal tract. The anterograde anatomical tracer BDA was injected into the leg and trunk regions of the left primary motor cortex to label corticospinal tract fibres in the spinal cord, shown in pink in the photographs. The top-right scheme shows the approximate pathways of the dorsal ascending tract and all descending tracts identified from refs 36 and 37. For monkeys Q2 and Q3, the area of maximal damage was reconstructed in two dimensions by identifying the border of the glia scar on evenly spaced sagittal sections spanning the entire dorsoventral extent of the lesioned spinal cord. b–e, For each monkey, confocal photographs show longitudinal sections of the lesioned spinal cord at specific dorsoventral levels, as indicated with dotted lines. In each photograph, the following anatomical elements are labelled: astrocytes (GFAP, grey), neural cell bodies (NeuN, cyan) and corticospinal tract axons (BDA, pink). The insets show high-resolution photographs of selected regions (white squares) of the same photograph that illustrates intact corticospinal tract axons above the injury, axon retraction bulbs directly above the lesion, and the absence of axons below the injury. Monkey Q2 displayed a small subset of spared corticospinal tract axons in the more dorsal aspect of the dorsolateral column. The asterisks indicate the location of the lesion. Overview scale bars, 500 μm. Inset scale bars, 50 μm. f, Gait cycles were extracted from locomotion recorded in monkeys Q2 and Q3 pre-lesion, during the first two weeks after the lesion, and at 99 days after the lesion. Analysis was only applied to gait cycles classified as steps, that is, gait cycles classified as limb paralysis or stumbling were not included. The number of analysed gait cycles is directly reported into the figure. The bar plots report the mean Euclidean distance between all the steps on a given recording day without stimulation and steps recorded before the lesion, computed in the entire space of the 26 kinematic parameters. The other bar plots report mean values of relevant kinematic parameters. This analysis illustrates the progressive yet incomplete recovery of locomotion. ***P < 0.001, Wilcoxon rank-sum test. Error bars, s.e.m.
Extended Data Figure 8 Restoration of leg locomotor movements during overground locomotion after the spinal cord lesion.
a, Representative sequences of locomotion along a straight corridor without stimulation and during brain-controlled stimulation recorded at 7 days and 13 days after the lesion for monkey Q2. Conventions are the same as in Fig. 4. The bar plots report the mean step height of the right (lesioned) leg during swing. *P < 0.05, Wilcoxon rank-sum test. Error bars, s.e.m. b, Snapshots extracted from video recordings showing a representative sequence of leg movements during one gait cycle without stimulation and during brain-controlled stimulation at 7 days post-injury for monkey Q2. The stick diagrams overlying the right (lesioned side) leg and the trajectory of the foot are colour-coded using the same colour scheme as in the stick diagram decomposition in a. Without stimulation, the monkey dragged the leg along the ground, whereas brain-controlled stimulation restored weight-bearing locomotion with plantar placement.
Extended Data Figure 9 Quantification of gait improvements and decoding accuracy during brain-controlled stimulation after the spinal cord lesion.
a, Two successive gait cycles performed during locomotion on a treadmill without stimulation and during brain-controlled stimulation in monkey Q2 at 6 days and 14 days post-lesion, and in monkey Q3 at 16 days post-injury. Conventions are the same as in Fig. 4. In addition, the average foot trajectories calculated over all the recorded gait cycles are displayed for each experimental condition including during pre-lesion locomotion, illustrating the marked improvement of foot movements during brain-controlled stimulation. b, Bar plot reporting the mean values of the total excursion of the angle, step height and foot trajectory area for monkeys Q2 and Q3 during locomotion pre-lesion and post-lesion without stimulation and with brain-controlled stimulation. Analysis was only applied to gait cycles classified as steps, that is, gait cycles classified as limb paralysis or stumbling were not included. (For monkey Q2: pre-lesion n = 294, day 6 post-lesion no stimulation n = 6, brain control n = 12, day 14 no stimulation n = 39, brain control n = 93. For monkey Q3: pre-lesion n = 185, day 16 post-injury no stimulation n = 98, brain control n = 31.) *P < 0.05, **P < 0.01, ***P < 0.001, Wilcoxon rank-sum test. Error bars, s.e.m. c, Bar plots reporting the capacity of the monkeys to sustain walking at the imposed treadmill belt speed. The functional score is computed as the percentage of regular steps in which the animal is able to walk at the treadmill belt speed; that is, the animal does not bump into the back of the treadmill. Gait cycles classified as hops or stumbling were not included. *P < 0.05, ***P < 0.001, bootstrap. Error bars, s.e.m. d, Decoding accuracy increases during recovery after the spinal cord lesion. Decoder confusion matrices calculated reporting the accuracy of the real-time decoders and chance level decoder during brain-controlled stimulation for monkey Q2 at day 6 (n = 76 foot-strikes, n = 74 foot-offs) and day 14 post-injury (n = 264 foot-strikes, n = 264 foot-offs) and for monkey Q3 at 16 days post-injury (n = 319 foot-strikes, n = 321 foot-offs). The tolerance window was set at ±125 ms. The bar plots report the normalized mutual information calculated for the real-time decoders compared to random decoders and chance level decoders. ***,**P < 0.001 and P < 0.01, respectively. Bootstrap. Error bars, s.e.m. e, Top panels, from left to right: mean event rate, modulation depth and preferred direction for the neuronal spiking signal recorded obtained by regressing spike rates against the phase of the gait cycle for monkeys Q1 to Q3. Preferred direction was defined as the angle for which the fitted tuning function was at a maximum. Bottom panels, from left to right: mean absolute single-electrode difference for mean event rates, modulation depths and preferred directions between two consecutive sessions shown in the top panels. Analysis shows substantial changes both before and after the spinal-cord lesion. Nevertheless, the rate of change between the last pre-lesion and the first post-lesion sessions was substantially higher than between any two other session pairs, thus indicating increased level of plasticity following spinal cord lesion. **P < 0.01, ***P < 0.001, Monte Carlo, Wilcoxon rank-sum test, signed Wilcoxon rank-sum test and bootstrap. Error bars, s.e.m.
Extended Data Figure 10 The temporal structure and features of stimulation determine the quantity and quality of steps.
a, Two successive gait cycles performed during locomotion on a treadmill during brain-controlled stimulation with optimal and suboptimal temporal structures at 6 days post-injury, and during continuous stimulation at 14 days post-lesion for monkey Q2. The gait cycles were classified as suboptimal temporal structures when stimulation occurred outside a ±125 ms tolerance window. Conventions are the same as in Fig. 4. b, Bar plots reporting the quantity of steps calculated during locomotion with optimal and suboptimal temporal structures over a range of stimulation frequencies and during continuous stimulation for monkeys Q2 and Q3 at 6 days, 14 days and 16 days post-injury. *P < 0.05, **P < 0.01, ***P < 0.001, bootstrap. Error bars, s.e.m. c, Bar plots reporting the quality of stepping for the same conditions as in b. The quality of stepping was measured as the mean Euclidean distance between pre-lesion and post-lesion gait cycles calculated in the kinematic space defined by the 26 gait parameters, as reported in Fig. 4. These results show that the optimal temporal structure leads to an increased number of steps and improved quality of stepping compared to sub-optimal temporal structures. Moreover, brain-controlled stimulation with both optimal and suboptimal temporal structure promoted markedly improved locomotor performance compared to continuous stimulation delivered with the same stimulation features. These results also highlight the ability to optimize locomotor performance when tuning the stimulation frequency. *P < 0.05, **P < 0.01, ***P < 0.001, Wilcoxon rank-sum test. Error bars, s.e.m.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data and Supplementary Tables 1-3. (PDF 669 kb)
Technical design and therapeutic effects of the brain–spinal interface
This video explains the design of the spinal cord stimulation system and brain decoding algorithms. The ability of the brain–spinal interface to modulate extension and flexion movements of the leg during continuous locomotion is then illustrated in intact monkeys. Finally, the video shows the recovery of functional leg movements during locomotion on a treadmill and overground in two monkeys with a spinal cord injury. (MP4 28900 kb)
Rights and permissions
About this article
Cite this article
Capogrosso, M., Milekovic, T., Borton, D. et al. A brain–spine interface alleviating gait deficits after spinal cord injury in primates. Nature 539, 284–288 (2016). https://doi.org/10.1038/nature20118
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature20118
This article is cited by
-
Dual electrical stimulation at spinal-muscular interface reconstructs spinal sensorimotor circuits after spinal cord injury
Nature Communications (2024)
-
Brain–computer interface: trend, challenges, and threats
Brain Informatics (2023)
-
Spinal cord injury: molecular mechanisms and therapeutic interventions
Signal Transduction and Targeted Therapy (2023)
-
Epidural stimulation of the cervical spinal cord for post-stroke upper-limb paresis
Nature Medicine (2023)
-
A digital bridge to reverse paralysis
Cell Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.