Abstract
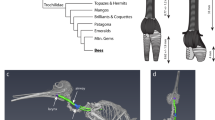
From complex songs to simple honks, birds produce sounds using a unique vocal organ called the syrinx1,2. Located close to the heart at the tracheobronchial junction, vocal folds or membranes attached to modified mineralized rings vibrate to produce sound1,2,3,4,5,6,7. Syringeal components were not thought to commonly enter the fossil record6, and the few reported fossilized parts of the syrinx are geologically young8,9,10,11 (from the Pleistocene and Holocene (approximately 2.5 million years ago to the present)). The only known older syrinx is an Eocene specimen that was not described or illustrated12. Data on the relationship between soft tissue structures and syringeal three-dimensional geometry are also exceptionally limited5. Here we describe the first remains, to our knowledge, of a fossil syrinx from the Mesozoic Era, which are preserved in three dimensions in a specimen from the Late Cretaceous (approximately 66 to 69 million years ago) of Antarctica. With both cranial and postcranial remains, the new Vegavis iaai specimen is the most complete to be recovered from a part of the radiation of living birds (Aves). Enhanced-contrast X-ray computed tomography (CT) of syrinx structure in twelve extant non-passerine birds, as well as CT imaging of the Vegavis and Eocene syrinxes, informs both the reconstruction of ancestral states in birds and properties of the vocal organ in the extinct species. Fused rings in Vegavis form a well-mineralized pessulus, a derived neognath bird feature, proposed to anchor enlarged vocal folds or labia5. Left-right bronchial asymmetry, as seen in Vegavis, is only known in extant birds with two sets of vocal fold sound sources. The new data show the fossilization potential of the avian vocal organ and beg the question why these remains have not been found in other dinosaurs. The lack of other Mesozoic tracheobronchial remains, and the poorly mineralized condition in archosaurian taxa without a syrinx, may indicate that a complex syrinx was a late arising feature in the evolution of birds, well after the origin of flight and respiratory innovations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Huxley, T. H. A Manual of the Anatomy of Vertebrate Animals (Appleton, 1872)
Beddard, F. E. The Structure and Classification of Birds (Longmans, Green & Co, 1898)
Paulsen, K. Das Prinzip der Stimmbildung in der Wirbeltierreihe und beim Menschen (Akademische Verlagsgesellschaft, 1967)
Ames, P. L. The morphology of the syrinx in passerine birds. Bulletin of the Peabody Museum of Natural History Vol. 37 (Yale University, 1971)
King, A. S. Functional anatomy of the syrinxin Form and Function in Birds (eds King, A. S. & McLelland, J. ) 4, 105–192. (Academic Press, 1989)
ten Cate, C. Birdsong and Evolution in Nature’s Music. The Science of Birdsong (eds Marler, P. M. and Slabbekoorn, H. ) 296–317 (Elsevier, 2004)
Goller, F. & Larsen, O. N. A new mechanism of sound generation in songbirds. Proc. Natl Acad. Sci. USA 94, 14787–14791 (1997)
Woolfenden, G. E. A Pleistocene avifauna from Rock Spring, Florida. Wilson Bull. 71, 183–187 (1959)
Olson, S. L. & James, H. F. Descriptions of thirty-two new species of birds from the Hawaiian Islands: Part I. non-passeriformes. Ornithol. Monogr. 45, 38–42 (1991)
Worthy, T. & Holdaway, R. N. The Lost World of the Moa: Prehistoric Life of New Zealand. (Indiana Univ. Press, 2002)
Degrange, F. J., Tambussi, C. P., Taglioretti, M. L., Dondas, A. & Scaglia, F. A new Mesembriornithinae (Aves, Phorusrhacidae) provides new insights into the phylogeny and sensory capabilities of terror birds. J. Vert. Paleont. 35, e912656 (2015)
Olson, S. L. & Feduccia, A. Presbyornis and the origin of the Anseriformes (Aves: Charadriomorphae). Smithson. Contrib. Zool. 323, 1–24 (1980)
Senter, P. Voices of the past: a review of Paleozoic and Mesozoic animal sounds. Hist. Biol. 20, 255–287 (2008)
Weishampel, D. B. Acoustic analyses of potential vocalization in lambeosaurine dinosaurs (Reptilia: Ornithischia). Paleobiology 7, 252–261 (1981)
Evans, D. C. Nasal cavity homologies and cranial crest function in lambeosaurine dinosaurs. Paleobiology 32, 109–125 (2006)
Tattersall, I. Communication and human uniqueness in The Evolution of Social Communication in Primates 219–227 (Springer, 2014)
Veselka, N. et al. A bony connection signals laryngeal echolocation in bats. Nature 463, 939–942 (2010)
Clarke, J. A., Tambussi, C. P., Noriega, J. I., Erickson, G. M. & Ketcham, R. A. Definitive fossil evidence for the extant avian radiation in the Cretaceous. Nature 433, 305–308 (2005)
Gignac, P. M. et al. Diffusible iodine-based contrast-enhanced computed tomography (diceCT): an emerging tool for rapid, high-resolution, 3-D imaging of metazoan soft tissues. J. Anat. 228, 889–909 (2016)
Düring, D. N. et al. The songbird syrinx morphome: a three-dimensional, high-resolution, interactive morphological map of the zebra finch vocal organ. BMC Biol. 11, 1 (2013)
Goller, F. & Riede, T. Integrative physiology of fundamental frequency control in birds. J. Physiol. (Paris) 107, 230–242 (2013)
Picasso, M. B. J. & Carril, J. The peculiar syrinx of Rhea Americana (Greater Rhea, Palaeognathae). Vertebr. Zool. 63, 321–327 (2013)
Johnsgard, P. A. Tracheal anatomy of the Anatidae and its taxonomic significance. Wildfowl 12, 58–69 (1961)
Johnsgard, P. A. Comparative behavior and relationships of the eiders. Condor 66, 113–129 (1964)
Riede, T., Eliason, C. M., Miller, E. H., Goller, F. & Clarke, J. A. Coos, booms, and hoots: the evolution of closed-mouth vocal behavior in birds. Evolution 70, 1734–1746 (2016)
Mayr, G. A new raptor-like bird from the Lower Eocene of North America and Europe. Senckenbergiana lethaea 80, 59–65 (2000)
Clarke, J. A., Ksepka, D. T., Smith, N. A. & Norell, M. Combined phylogenetic analysis of a new North American fossil species confirms widespread Eocene distribution for stem rollers (Aves, Coracii). Zool. J. Linn. Soc. 157, 586–611 (2009)
Xu, X. et al. An integrative approach to understanding bird origins. Science 346, 1253293 (2014)
Balanoff, A. M., Bever, G. S., Rowe, T. B. & Norell, M. A. Evolutionary origins of the avian brain. Nature 501, 93–96 (2013)
Dunbar, R. I. & Shultz, S. Evolution in the social brain. Science 317, 1344–1347 (2007)
Acknowledgements
This project was funded by the Gordon and Betty Moore Foundation (grant GBMF4498; J.A.C., T.R. and F.G.), as well as the National Science Foundation (OPP ANT-1141820, OPP 0927341 and EAR 1355292; J.A.C.), C. Burke and the Agencia Nacional de Promoción Científica y Técnica (PICT 2010-066; F.E.N). The Instituto Antártico Argentino (IAA), G. M. Robles, W. J. Zinsmeister and especially C. A. Rinaldi, E. B. Olivero, and the Fuerza Aérea Argentina provided key support for fieldwork in 1993.
Author information
Authors and Affiliations
Contributions
D.R.M. and F.J.M. collected the fossil specimen and contributed geological data. M.P.I. and S.C. contributed to the fossil specimen preparation and study. T.R., F.G., Z.L. and J.A.C. designed the study of the syrinx and collected primary data. Z.L. designed the enhanced contrast CT protocol and collected extant CT data. J.A.C. discovered the fossil syrinx remains and designed the project with F.E.N., S.C., T.R. and F.G. J.A.C., Z.L., F.A., F.E.N., T.R., F.G. and S.C. conducted morphological study of the specimen.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information
Nature thanks P. O’Connor and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 Photographs of elements from the new Vegavis iaai specimen from Vega Island, Antarctica (MACN-PV 19.748) prepared from the primary block.
a–d, Coracoid(a), caudal vertebra (b), (from top to bottom) distal radius, ulna with radiale in articulation, radiale and ulnare, and manual phalanx III:1 (c), pedal phalanx (d), scapula (e), femur (f), tibiotarsus (g). Scale bar, 1 cm.
Extended Data Figure 2 Images from X-ray computed tomography of selected elements of the new Vegavis iaai specimen (MACN-PV 19.748).
a, Coracoid (acr, acrocoracoid; gl, glenoid facet; pp, procoracoid process; scc, scapular cotyla). b, Proximal tibia (cnc, cranial cnemial crest). c, Pterygoid (bpt, basipterygoid articular facet; pat, palatine articular facet; qut, quadrate articular facet). d, Fibula (fi). e, Radialae and ulnare. f, Caudal mandible. g, Proximal humerus (dep, depression; dc, deltopectoral crest; ci, captal incisure; fo, fossa). h, Femur (os, ovoid scar). Scale bar, 1 cm.
Extended Data Figure 3 Polarornis gregorii holotype specimen from Seymour Island, Antarctic Peninsula.
The specimen was damaged during original preparation and the existence of the previously described braincase and quadrate as well as morphologies from the skull and tibial shaft cannot be confirmed.
Extended Data Figure 4 Comparison of elements in common between the Polarornis gregorii holotype specimen from Seymour Island (top) and the new specimen of Vegavis iaai (MACN-PV 19.748) (bottom).
a, b, Tibiotarsus (a) and femur (b). The asterisk indicates the prominent muscular ridge on the femur present in the Vegavis iaai holotype and the newly referred specimen (MACN-PV 19.748), but not seen in the Polarornis holotype. Scale bar, 1 cm.
Extended Data Figure 5 Comparison of select elements in common between the Vegavis iaai holotype and the new specimen (MACN-PV 19.748).
Top, the circular depression on the proximal femur used in the diagnosis of Vegavis seen in the new specimen18. Bottom, the humeral crest (lateral to the capital ridge) also mentioned in that diagnosis of Vegavis iaai are shown. Although both of these characters show a complex distribution in Aves, and are not restricted to the species, their combination is unique.
Extended Data Figure 6 The pterygoid in Vegavis iaai specimen MACN-PV 19.748 (right) compared to that of Gavia immer (left) highlighting the prominent basipterygoid facet in the Vegavis iaai referred specimen.
See also Fig. 1, Extended Data Fig. 2 and Supplementary Information. Scale bar, 1 cm.
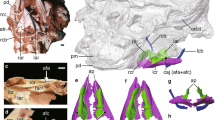
Extended Data Figure 7 Vegavis iaai (MACN-PV 19.748) syrinx with position-based identities labelled.
See also Fig. 2 and Supplementary Table 3.
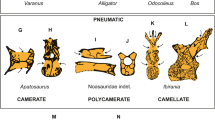
Extended Data Figure 8 Morphological comparison of the syrinx in the fossils and enhanced contrast CT images of avian and outgroup exemplars. Key features discussed in the text are illustrated.
The numbers (−1, 0, 1) reference the position schema described in the main text, Supplementary Information, Supplementary Tables 3 and 4, Extended Data Fig. 7. Enlarged images are available at http://www.jsg.utexas.edu/syrinx-evolution/ and this image website is also available at Data Dryad as a zipped file at http://dx.doi.org/10.5061/dryad.50n8j.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary Tables 1-4 and additional references. (PDF 702 kb)
Supplementary Figures
This file contains Supplementary Figures 1-2. (PDF 262 kb)
Rights and permissions
About this article
Cite this article
Clarke, J., Chatterjee, S., Li, Z. et al. Fossil evidence of the avian vocal organ from the Mesozoic. Nature 538, 502–505 (2016). https://doi.org/10.1038/nature19852
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature19852
This article is cited by
-
A juvenile bird with possible crown-group affinities from a dinosaur-rich Cretaceous ecosystem in North America
BMC Ecology and Evolution (2024)
-
An ankylosaur larynx provides insights for bird-like vocalization in non-avian dinosaurs
Communications Biology (2023)
-
Common evolutionary origin of acoustic communication in choanate vertebrates
Nature Communications (2022)
-
A new passeriform (Aves: Passeriformes) from the early Oligocene of Poland sheds light on the beginnings of Suboscines
Journal of Ornithology (2021)
-
The hummingbird syrinx morphome: a detailed three-dimensional description of the black jacobin’s vocal organ
BMC Zoology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.