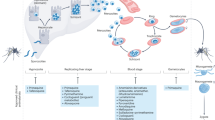
Abstract
Antimalarial drugs have thus far been chiefly derived from two sources—natural products and synthetic drug-like compounds. Here we investigate whether antimalarial agents with novel mechanisms of action could be discovered using a diverse collection of synthetic compounds that have three-dimensional features reminiscent of natural products and are underrepresented in typical screening collections. We report the identification of such compounds with both previously reported and undescribed mechanisms of action, including a series of bicyclic azetidines that inhibit a new antimalarial target, phenylalanyl-tRNA synthetase. These molecules are curative in mice at a single, low dose and show activity against all parasite life stages in multiple in vivo efficacy models. Our findings identify bicyclic azetidines with the potential to both cure and prevent transmission of the disease as well as protect at-risk populations with a single oral dose, highlighting the strength of diversity-oriented synthesis in revealing promising therapeutic targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wells, T. N. Discovering and developing new medicines for malaria control and elimination. Infect. Disord. Drug Targets 13, 292–302 (2013)
Flannery, E. L., Chatterjee, A. K. & Winzeler, E. A. Antimalarial drug discovery—approaches and progress towards new medicines. Nat. Rev. Microbiol. 11, 849–862 (2013)
Ariey, F. et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505, 50–55 (2014)
Campo, B., Vandal, O., Wesche, D. L. & Burrows, J. N. Killing the hypnozoite—drug discovery approaches to prevent relapse in Plasmodium vivax. Pathog. Glob. Health 109, 107–122 (2015)
Hameed P, S. et al. Triaminopyrimidine is a fast-killing and long-acting antimalarial clinical candidate. Nat. Commun. 6, 6715 (2015)
Jiménez-Díaz, M. B. et al. Improved murine model of malaria using Plasmodium falciparum competent strains and non-myelodepleted NOD-scid IL2Rγnull mice engrafted with human erythrocytes. Antimicrob. Agents Chemother. 53, 4533–4536 (2009)
Phillips, M. A. et al. A long-duration dihydroorotate dehydrogenase inhibitor (DSM265) for prevention and treatment of malaria. Sci. Transl. Med. 7, 296ra111 (2015)
Roberts, L. & Enserink, M. Malaria. Did they really say ... eradication? Science 318, 1544–1545 (2007)
Rottmann, M. et al. Spiroindolones, a potent compound class for the treatment of malaria. Science 329, 1175–1180 (2010)
Gamo, F.-J. et al. Thousands of chemical starting points for antimalarial lead identification. Nature 465, 305–310 (2010)
Guiguemde, W. A. et al. Chemical genetics of Plasmodium falciparum. Nature 465, 311–315 (2010)
Meister, S. et al. Imaging of Plasmodium liver stages to drive next-generation antimalarial drug discovery. Science 334, 1372–1377 (2011)
Comer, E. et al. Diversity-oriented synthesis-facilitated medicinal chemistry: toward the development of novel antimalarial agents. J. Med. Chem. 57, 8496–8502 (2014)
Heidebrecht, R. W. Jr et al. Diversity-oriented synthesis yields a novel lead for the treatment of malaria. ACS Med. Chem. Lett. 3, 112–117 (2012)
Lukens, A. K. et al. Diversity-oriented synthesis probe targets Plasmodium falciparum cytochrome b ubiquinone reduction site and synergizes with oxidation site inhibitors. J. Infect. Dis. 211, 1097–1103 (2015)
Dancík, V., Seiler, K. P., Young, D. W., Schreiber, S. L. & Clemons, P. A. Distinct biological network properties between the targets of natural products and disease genes. J. Am. Chem. Soc. 132, 9259–9261 (2010)
Burke, M. D. & Schreiber, S. L. A planning strategy for diversity-oriented synthesis. Angew. Chem. Int. Edn Engl. 43, 46–58 (2004)
Marcaurelle, L. A. et al. An aldol-based build/couple/pair strategy for the synthesis of medium- and large-sized rings: discovery of macrocyclic histone deacetylase inhibitors. J. Am. Chem. Soc. 132, 16962–16976 (2010)
Painter, H. J., Morrisey, J. M., Mather, M. W. & Vaidya, A. B. Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature 446, 88–91 (2007)
McNamara, C. W. et al. Targeting Plasmodium PI(4)K to eliminate malaria. Nature 504, 248–253 (2013)
Ghidelli-Disse, S. et al. Identification of Plasmodium PI4 kinase as target of MMV390048 by chemoproteomics. Malar. J. 13 (suppl. 1), 38 (2014)
Sharma, A. & Sharma, A. Plasmodium falciparum mitochondria import tRNAs along with an active phenylalanyl-tRNA synthetase. Biochem. J. 465, 459–469 (2015)
Pham, J. S. et al. Aminoacyl-tRNA synthetases as drug targets in eukaryotic parasites. Int. J. Parasitol. 4, 1–13 (2014)
Herman, J. D. et al. The cytoplasmic prolyl-tRNA synthetase of the malaria parasite is a dual-stage target of febrifugine and its analogs. Sci. Transl. Med. 7, 288ra77 (2015)
Hussain, T., Yogavel, M. & Sharma, A. Inhibition of protein synthesis and malaria parasite development by drug targeting of methionyl-tRNA synthetases. Antimicrob. Agents Chemother. 59, 1856–1867 (2015)
Novoa, E. M. et al. Analogs of natural aminoacyl-tRNA synthetase inhibitors clear malaria in vivo. Proc. Natl Acad. Sci. USA 111, E5508–E5517 (2014)
Hoepfner, D. et al. Selective and specific inhibition of the Plasmodium falciparum lysyl-tRNA synthetase by the fungal secondary metabolite cladosporin. Cell Host Microbe 11, 654–663 (2012)
Istvan, E. S. et al. Validation of isoleucine utilization targets in Plasmodium falciparum. Proc. Natl Acad. Sci. USA 108, 1627–1632 (2011)
Bhatt, T. K. et al. A genomic glimpse of aminoacyl-tRNA synthetases in malaria parasite Plasmodium falciparum. BMC Genomics 10, 644 (2009)
Vaughan, A. M. et al. A transgenic Plasmodium falciparum NF54 strain that expresses GFP-luciferase throughout the parasite life cycle. Mol. Biochem. Parasitol. 186, 143–147 (2012)
Vaughan, A. M. et al. Plasmodium falciparum genetic crosses in a humanized mouse model. Nat. Methods 12, 631–633 (2015)
Chang, H.-H. et al. Persistence of Plasmodium falciparum parasitemia after artemisinin combination therapy: evidence from a randomized trial in Uganda. Sci. Rep. 6, 26330 (2016)
Joice, R. et al. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci. Transl. Med. 6, 244re5 (2014)
Ding, X. C., Ubben, D. & Wells, T. N. A framework for assessing the risk of resistance for anti-malarials in development. Malar. J. 11, 292 (2012)
Dandapani, S. & Marcaurelle, L. A. Grand challenge commentary: Accessing new chemical space for ‘undruggable’ targets. Nat. Chem. Biol. 6, 861–863 (2010)
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009)
Lowe, J. T. et al. Synthesis and profiling of a diverse collection of azetidine-based scaffolds for the development of CNS-focused lead-like libraries. J. Org. Chem. 77, 7187–7211 (2012)
Baragaña, B. et al. A novel multiple-stage antimalarial agent that inhibits protein synthesis. Nature 522, 315–320 (2015)
Younis, Y. et al. 3,5-Diaryl-2-aminopyridines as a novel class of orally active antimalarials demonstrating single dose cure in mice and clinical candidate potential. J. Med. Chem. 55, 3479–3487 (2012)
Burrows, J. N., van Huijsduijnen, R. H., Möhrle, J. J., Oeuvray, C. & Wells, T. N. C. Designing the next generation of medicines for malaria control and eradication. Malar. J. 12, 187 (2013)
Ng, P. Y., Tang, Y., Knosp, W. M., Stadler, H. S. & Shaw, J. T. Synthesis of diverse lactam carboxamides leading to the discovery of a new transcription-factor inhibitor. Angew. Chem. Int. Edn Engl. 46, 5352–5355 (2007)
Yu, C. et al. High-throughput identification of genotype-specific cancer vulnerabilities in mixtures of barcoded tumor cell lines. Nat. Biotechnol. 34, 419–423 (2016)
Jaccard, P. Lois de distribution florale dans la zone alpine. Bull. Soc. Vaud. Sci. Nat. 38, 69–130 (1902)
Rogers, D. & Hahn, M. Extended-connectivity fingerprints. J. Chem. Inf. Model. 50, 742–754 (2010)
Pipeline Pilot v. 8.5.0.200; http://cscenter.pbsci.ucsc.edu:9944/ (Accelrys Software Inc., 2011)
R Core Development Team. R: A Language and Environment for Statistical Computing v. 3.0 ; http://www.R-project.org/ (R Foundation for Statistical Computing, Vienna, Austria, 2013)
Khetani, S. R. & Bhatia, S. N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 26, 120–126 (2008)
March, S. et al. A microscale human liver platform that supports the hepatic stages of Plasmodium falciparum and vivax. Cell Host Microbe 14, 104–115 (2013)
March, S. et al. Micropatterned coculture of primary human hepatocytes and supportive cells for the study of hepatotropic pathogens. Nat. Protocols 10, 2027–2053 (2015)
Rheinwald, J. G. & Green, H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6, 331–343 (1975)
Ponnudurai, T. et al. Infectivity of cultured Plasmodium falciparum gametocytes to mosquitoes. Parasitology 98, 165–173 (1989)
Guguen-Guillouzo, C. et al. High yield preparation of isolated human adult hepatocytes by enzymatic perfusion of the liver. Cell Biol. Int. Rep. 6, 625–628 (1982)
Dembele, L. et al. Towards an in vitro model of Plasmodium hypnozoites suitable for drug discovery. PLoS One 6, e18162 (2011)
Mazier, D. et al. Complete development of hepatic stages of Plasmodium falciparum in vitro. Science 227, 440–442 (1985)
Duffy, S. & Avery, V. M. Identification of inhibitors of Plasmodium falciparum gametocyte development. Malar. J. 12, 408 (2013)
Stone, W. J. R. et al. A scalable assessment of Plasmodium falciparum transmission in the standard membrane-feeding assay, using transgenic parasites expressing green fluorescent protein-luciferase. J. Infect. Dis. 210, 1456–1463 (2014)
Manary, M. J. et al. Identification of pathogen genomic variants through an integrated pipeline. BMC Bioinformatics 15, 1–14 (2014)
Ross, L. S. et al. In vitro resistance selections for Plasmodium falciparum dihydroorotate dehydrogenase inhibitors give mutants with multiple point mutations in the drug-binding site and altered growth. J. Biol. Chem. 289, 17980–17995 (2014)
Hansen, M. et al. Inhibitor binding in a class 2 dihydroorotate dehydrogenase causes variations in the membrane-associated N-terminal domain. Protein Sci. 13, 1031–1042 (2004)
Burke, J. E. et al. Structures of PI4KIIIβ complexes show simultaneous recruitment of Rab11 and its effectors. Science 344, 1035–1038 (2014)
Burke, J. E. & Williams, R. L. Dynamic steps in receptor tyrosine kinase mediated activation of class IA phosphoinositide 3-kinases (PI3K) captured by H/D exchange (HDX-MS). Adv. Biol. Regul. 53, 97–110 (2013)
Arnold, K., Bordoli, L., Kopp, J. & Schwede, T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22, 195–201 (2006)
Benkert, P., Biasini, M. & Schwede, T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27, 343–350 (2011)
Biasini, M. et al. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 42, W252–W258 (2014)
Jacobson, M. P. et al. A hierarchical approach to all-atom protein loop prediction. Proteins 55, 351–367 (2004)
Sherlin, L. D. et al. Chemical and enzymatic synthesis of tRNAs for high-throughput crystallization. RNA 7, 1671–1678 (2001)
Cestari, I. & Stuart, K. A spectrophotometric assay for quantitative measurement of aminoacyl-tRNA synthetase activity. J. Biomol. Screen. 18, 490–497 (2013)
Angulo-Barturen, I. et al. A murine model of falciparum-malaria by in vivo selection of competent strains in non-myelodepleted mice engrafted with human erythrocytes. PLoS One 3, e2252 (2008)
Ekland, E. H., Schneider, J. & Fidock, D. A. Identifying apicoplast-targeting antimalarials using high-throughput compatible approaches. FASEB J. 25, 3583–3593 (2011)
Joice, R. et al. Inferring developmental stage composition from gene expression in human malaria. PLOS Comput. Biol. 9, e1003392 (2013)
Ruecker, A. et al. A male and female gametocyte functional viability assay to identify biologically relevant malaria transmission-blocking drugs. Antimicrob. Agents Chemother. 58, 7292–7302 (2014)
Zeeman, A.-M. et al. KAI407, a potent non-8-aminoquinoline compound that kills Plasmodium cynomolgi early dormant liver stage parasites in vitro. Antimicrob. Agents Chemother. 58, 1586–1595 (2014)
Lukens, A. K. et al. Harnessing evolutionary fitness in Plasmodium falciparum for drug discovery and suppressing resistance. Proc. Natl Acad. Sci. USA 111, 799–804 (2014)
Acknowledgements
This work was supported in part by the Bill and Melinda Gates Foundation (grant OPP1032518 to S.L.S., grant OPP1054480 to E.A.W. and D.F.W., grant OPP1023607 to S.N.B.), the Global Health Innovative Technology Fund (grant G2014-107 to S.L.S.), Medicines for Malaria Venture and the Wellcome Trust (grant WT078285 to C.H.M.K.), a New Investigator and Open Operating Grant from Canadian Institute of Health Research (grant FRN 142393 to J.E.B.) and Medicines for Malaria Venture (grant 12-2400 to V.M.A.). S.L.S. is an Investigator at the Howard Hughes Medical Institute. Mi.M. was supported by a fellowship from the National Science Foundation (DGE1144152). The authors thank R. Elliott, K. Duncan, and O. Vandal as well as J. Burrows, J. Duffy, F. Escudié and colleagues for discussions and access to invaluable scientific and experimental resources; K. Emmith for assistance with data processing and management; I. Goldowitz for assistance with establishing a gametocyte assay; N. van der Werff for technical assistance with the P. cynomolgi assay; J. Kotz and B. Melillo for discussions and assistance with the manuscript; J. Pu, M. Leighty, B. Braibant, S. LeQuement and J. Beaudoin for assistance with compound synthesis; E. Garcia-Rivera for assistance with molecular modelling; A. Hakura for performing the Ames test; Broad Institute Comparative Medicine Platform and Facility for assistance with animal studies; and the Broad Institute Compound Management and analytical teams for assistance with compound access and characterization. We also acknowledge WuXi AppTec and ChemPartner Co., Ltd for in vitro and in vivo pharmacokinetics assays, and Cyprotek for the phototoxicity analysis. P. falciparum scDHODH transgenic strain was a gift from A. B. Vaidya, P. falciparum 3D7HLH strain from D. Fidock and P. falciparum NF54HT-GFP-luc from S. H. Kappe.
Author information
Authors and Affiliations
Contributions
The author contributions are detailed in the Supplementary Information.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Three screening-hit series yield new compound scaffolds against known targets.
a–d, BRD0026 exhibits the same mode of action as NITD609 and showed moderate in vitro potency against asexual (EC50 = 0.346 μM) and late-sexual (EC50 = 1.98 μM) blood stages of the parasites and exhibited reduced potency against P. falciparum NITD609R (EC50 = 1.77 μM), a transgenic strain carrying a point mutation in P. falciparum ATPase4 (ref. 9). P. falciparum ATPase4 is the presumed molecular target of NITD609 (ref. 9). a, b, Three of the eight possible stereoisomers (R,S,R; S,S,S; and R,S,S) of BRD0026 have activity. c, Initial characterization of BRD0026 showed good solubility in PBS and low cytotoxicity. d, Treatment with BRD0026 resulted in a rapid increase in the parasite cytosolic Na+ concentration, while artesunate- or mefloquine-treated parasites maintained a constant cytosolic Na+ concentration. This result suggests that parasites treated with BRD0026 are not able to counter the influx of Na+ by actively extruding the cation, similar to the proposed mechanism for NITD609 (data are mean ± s.d.; two biological and two technical replicates). e–h,
BRD7539 targets and inhibits P. falciparum DHODH. BRD7539 showed excellent in vitro potency against liver-stages (EC50 = 0.015 μM) and asexual blood-stages (EC50 = 0.010 μM) of the parasite, conferring markedly reduced potency against PfscDHODH19. This strain heterologously expresses the cytosolic S. cerevisiae DHODH, which does not require ubiquinone as an electron acceptor. Thus, this transgenic strain is resistant to inhibitors of mitochondrial electron transport chain functions19. BRD7539 was tested against three different P. falciparum strains with mutations in mitochondrial genes targeted by other antimalarial agents: (i) TM90C6B strain, containing a point mutation in the quinol oxidase domain of P. falciparum cytochrome b (Qo site) and resistant to atovaquone15; (ii) a P. falciparum CYTbG33V mutant strain, selected against IDI5994 and containing a point mutation in the quinone reductase site of P. falciparum cytochrome b (Qi site)15; and (iii) a P. falciparum DHODHE182D mutant strain, selected against Genz-666136 and containing a point mutation in the P. falciparum DHODH gene73. BRD7539 exhibited an approximately 59-fold shift in potency against the P. falciparum DHODHE182D strain, whereas potency was unaffected in the TM90C6B and P. falciparum CYTbG33V strains. BRD7539 also inhibits recombinant P. falciparum DHODH in an in vitro biochemical assay (IC50 = 0.033 μM) but not the human orthologue. Altogether, these results demonstrate that BRD7539 targets P. falciparum DHODH. e, f, Only two (S,S,S and R,S,S) of eight possible stereoisomers of BRD7539 showed activity. g, In vitro growth inhibition assays showed no change in activity in P. falciparum CYTbG33V and TM90C6B strains but exhibited a tenfold change in potency in P. falciparum DHODHE182D strain, indicating that BRD7539 targets P. falciparum DHODH but not P. falciparum cytochrome bc1. h, BRD7539 inhibited recombinant P. falciparum DHODH in vitro with an IC50 of 33 nM; no inhibition of the human orthologues was observed (data are mean ± s.d. for two biological and two technical replicates). i–m, BRD73842 targets and inhibits P. falciparum PI4K. BRD73842 showed excellent in vitro activity against asexual (EC50 = 0.069 μM), late-sexual blood-stage (EC50 = 0.643 μM) and liver-stage (EC50 = 0.459 μM) parasites. i, j, The structure of BRD73842 indicates the required stereochemistry for activity (R stereoisomer). k, Initial characterization of BRD73842 showed good solubility and limited cytotoxicity. To gain insight into the mechanism of action of BRD73842, two resistant P. falciparum lines were evolved against BRD73842 from four independent cultures (a total of over 4 × 109 inocula, see Extended Data Fig. 2a). After more than 3 months of drug pressure, the EC50 values increased approximately 10- to 20-fold. Two clones were obtained from each culture. Sequence analyses revealed that all clones contain non-synonymous SNVs in PF3D7_0509800, the locus that encodes P. falciparum PI4K (Supplementary Table 3). l, To confirm that PI4K is the molecular target of BRD73842, the compound was assayed against purified recombinant P. vivax PI4K protein. BRD73842 selectively inhibits the kinase activity of P. vivax PI4K (IC50 = 21 nM), but not human PI4K. P. falciparum PI4K has been identified as the molecular target of two recently described antimalarial compounds, KAI407 (ref. 20) and MMV048 (ref. 21).(data are mean ± s.d.; two biological and two technical replicates). m, The biphasic dose–response curve is a signature of P. falciparum PI4K inhibitors (data are mean ± s.d.; three biological and three technical replicates). The EC50 values reported in this study are derived from the first transition of the dose–response curves (indicated by arrow).
Extended Data Figure 2 Resistance selection of BRD38427 and BRD1095.
a, Over 3 months of intermittent and increasing resistance selection pressure of BRD73842 starting at 150 nM (EC99.9) or 0.5 μM (10× EC50) yielded two cultures showing a 13- to 16-fold EC50 shift. Two clonal lines from each culture were developed and subjected to whole-genome sequencing. b, Over 3 months of intermittent pressure of BRD1095 at 60 nM (EC99.9) or 150 nM (10 × EC50) yielded three cultures showing a 3- to 67-fold EC50 shift. Two clonal lines from each culture were developed and subjected to whole-genome sequencing.
Extended Data Figure 3 In vivo blood-stage efficacy study of BRD7929.
a, BRD7929 shows single-dose in vivo efficacy in a P. berghei model of malaria. CD-1 mice were inoculated intravenously with approximately 2 × 107 P. berghei (ANKA GFP-luc) blood-stage parasites intravenously 24 h before treatment and BRD7929 was administered as a single 50, 25, or 12.5 mg kg−1 dose orally at 0 h (n = 4 for each group, this study was conducted once). Infections were monitored using IVIS. A single 100 mg kg−1 dose of artesunate results in rapid suppression of parasites, but owing to its short half-life, the parasites re-emerge very quickly. A single 25 mg kg−1 dose of BRD7929 resulted in 100% cure of the infected animals. One in four animals treated with a single oral dose of 12.5 mg kg−1 showed recrudescence at 6 days after treatment, but all other animals administered with 12.5 mg kg−1 were also completely parasite-free for 30 days. To ensure that no viable parasites remained, approximately 100 μl of combined blood samples from the four animals treated with 25 mg kg−1 of BRD7929 was intravenously injected into two naive mice and parasitaemia was monitored for an additional 30 days. No parasites were detected, suggesting that BRD7929 achieved a sterile cure for P. berghei with a single oral dose of as low as 25 mg kg−1. The same colour scale is used for the all images; not all time-point images are shown here. b, Bioluminescent intensity was quantified from each mouse and plotted against time. The dotted horizontal line represents the mean bioluminescence intensity level obtained from all the animals before the parasite inoculation. c, BRD7929 shows single-dose in vivo efficacy in a P. falciparum huRBC NSG mouse blood-stage model. huRBC NSG mice were inoculated intravenously with approximately 1 × 107 P. falciparum 3D7HLH/BRD blood-stage parasites 48 h before treatment and BRD7929 was administered as a single 50, 25, 12.5 or 6.12 mg kg−1 dose orally at 0 h (n = 2 for each group, this study was conducted once). Infections were monitored using the IVIS. No recrudescence was observed at doses as low as a single 12.5 mg kg−1 of BRD7929 in the infected animals. To ensure that no viable parasites remained, approximately 350 μl of combined blood samples from the two animals treated with 12.5 mg kg−1 of BRD7929 was cultured in vitro and monitored for an additional 30 days. No parasites were detected, suggesting that BRD7929 achieved a sterile cure for P. falciparum 3D7HLH/BRD with a single oral dose as low as 12.5 mg kg−1 (see Fig. 4a). The same colour scale is used for the all images; not all time point images are shown. Images of mice treated with vehicle on days 11 and 20 are not shown, because the bioluminescent signal was too high to show in the same colour scale as other images.
Extended Data Figure 4 In vivo liver-stage efficacy study of BRD7929 in a mouse malaria model.
a, BRD7929 shows single-dose causal prophylaxis in a P. berghei liver-stage model. CD-1 mice were inoculated intravenously with approximately 1 × 105 freshly dissected P. berghei ANKA luc-GFP sporozoites freshly dissected from A. stephensi salivary glands and immediately treated with a single oral dose of BRD7929 (25, 5, 1 or 0.2 mg kg−1). Infections were monitored using IVIS; mice were monitored until day 30 to ensure complete cure. No recrudescence was observed at doses as low as a single 5 mg kg−1 of BRD7929 in the infected animals (n = 4 for each group, study conducted once). The same colour scale is used for the all images. Not all time point images are shown. b, Bioluminescent intensity was quantified from each mouse and plotted against time. c, BRD7929 shows single-dose causal prophylaxis in a P. berghei liver-stage model up to 3 days before infection and two days after infection. CD-1 mice were infected with P. berghei and infections were monitored as described in a. Single oral doses of BRD7929 (10 mg kg−1) were administered at days 5, 3, and 1 before infection (days −5, −3 and −1), on day 0, and on days 1 and 2 after infection (n = 4 for each group, this study was conducted once). All dosing regimens except for the day −5 dose offered complete protection from infection for 32 days, indicating that BRD7929 has potent causal prophylaxis activity. The same colour scale is used for all images. Not all time-point images are shown.
Extended Data Figure 5 In vivo liver-stage efficacy study of BRD7929 in a humanized mouse model.
a, BRD7929 shows single-dose in vivo efficacy in a P. falciparum huHep FRG-knockout mouse liver-stage model. huHep FRG knockout mice were inoculated intravenously with approximately 1 × 105 P. falciparum (NF54HT-GFP-luc) sporozoites and BRD7929 was administered as a single 10 mg kg−1 oral dose 1 day after inoculation (n = 2 for each group, this study was conducted once). Infections were monitored using IVIS. The same colour scale is used for all images. No increase in bioluminescence intensity level was observed from the mice treated with BRD7929 (see Fig. 4b). b, Blood samples were also collected from each mouse 7 days after inoculation (the first day of the blood stage) and analysed for the presence of the blood-stage transcripts PF3D7_0812600 (P. falciparum UCE) using qRT–PCR32 (two biological replicates for each group and three technical replicates for each biological sample). Each dot represents a technical replicate of a sample and each horizontal line represents a mean of technical replicates from each mouse. The presence of the blood-stage parasite specific transcripts was detected from the control (vehicle) mice, while no amplification of the marker was detected after 40 amplification cycles (Ct value = 40) from the mice treated with BRD7929. Primer efficiency and sensitivity of the primer pairs for P. falciparum UCE have a detection limit ranging between 10 and 100 transcript copies33. Approximately 110 μl of combined blood samples from the two treated animals was also cultured in vitro and monitored for an additional 30 days but viable parasites were not detected.
Extended Data Figure 6 In vivo transmission-stage efficacy study of BRD7929.
a, Oral doses of BRD7929 2 days before feeding mosquitoes upon infected mice resulted in complete blocking of transmission at 5 mg kg−1, and reduced transmission activity at 1.25 mg kg−1 and 0.31 mg kg−1 (n = 2 for each group, this study was conducted once). b, Mosquitoes fed on vehicle-treated mice showed heavy infection 1 week after feeding, while mosquitoes fed on treated mice showed no or very few oocysts in the midguts. Representative images are shown; scale bars, 100 μm. c, To confirm that BRD7929 eliminates mature gametocytes in the host circulation rather than killing gametes, zygotes or ookinetes in the mosquito midgut, CD-1 mice infected with P. berghei (parasitaemia between 11 to 19%) were first treated with BRD7929 (oral, 25 mg kg−1). Infected mice were then exposed to female A. stephensi mosquitoes for blood feeding 1, 4 or 10 days after the treatment. Blood samples were also obtained before the blood feedings to measure the plasma concentration of remaining BRD7929 (n = 2 for each group, this study was conducted once). No oocysts were found in midguts dissected from mosquitoes from all time points, whereas 896.5, 170.5 and 8.6 ng ml−1 of the compound remained in the circulation 1, 4 and 10 days after respectively treatment, respectively, suggesting that BRD7929 eliminated mature gametocytes in the mice. d–f, In vivo transmission-stage efficacy study of BRD7929 (humanized mouse model). huRBC NSG mice were infected with the blood-stage P. falciparum 3D7HLH/BRD for 2 weeks to allow the gametocytes to mature fully and were treated with a single oral dose of BRD7929 (12.5 mg kg−1). n = 2 for each group, this study was conducted once. Blood samples collected from vehicle- and BRD7929-treated mice were tested for the presence of gametocyte-specific transcripts using mature gametocyte marker (PF3D7_1438800; d) and immature gametocyte marker (PF3D7_1477700; e). PF3D7_1120200 (P. falciparum UCE), a constitutively expressed gene, was used as a positive control marker for parasite detection (f). Data are mean ± s.d.; three technical replicates for each biological sample.
Extended Data Figure 7 Safety and resistance propensity profiling of the bicyclic azetidine series.
a, Results of in vitro cytotoxicity, phototoxicity and CYP inhibition assays. *Phototoxicity was assessed using the NIH 3T3 neutral red assay at Cyprotec; †CYP1A, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP3A; ‡CYP1A, CYP2C9, CYP2D6, CYP3A. b, Histopathology analysis of mice treated with a high dose (100 mg kg−1) of BRD7929. CD-1 mice were orally treated with 100 mg kg−1 BRD7929 and organs were collected 10 days after treatment. No significant tissue damage was detected. Representative images are shown here. Scale bars, 200 μm. c, d, Measurement of the minimal inoculum for resistance of BRD7929. Cultures containing various numbers of inoculum (1 × 105–1 × 109) were exposed to a constant level of drug pressure (EC90). Parasites developed resistance to atovaquone at the lowest inoculum of 1 × 107 but not to BRD7929.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary Tables 1-6 and additional references. (PDF 3188 kb)
Rights and permissions
About this article
Cite this article
Kato, N., Comer, E., Sakata-Kato, T. et al. Diversity-oriented synthesis yields novel multistage antimalarial inhibitors. Nature 538, 344–349 (2016). https://doi.org/10.1038/nature19804
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature19804
This article is cited by
-
Beyond PI3Ks: targeting phosphoinositide kinases in disease
Nature Reviews Drug Discovery (2023)
-
Antimalarial drug discovery: progress and approaches
Nature Reviews Drug Discovery (2023)
-
Potent acyl-CoA synthetase 10 inhibitors kill Plasmodium falciparum by disrupting triglyceride formation
Nature Communications (2023)
-
Diversity-oriented synthesis encoded by deoxyoligonucleotides
Nature Communications (2023)
-
Genomic analysis of single nucleotide polymorphisms in malaria parasite drug targets
Parasites & Vectors (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.