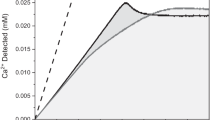
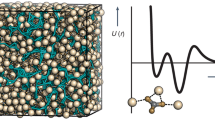
Abstract
Crystalline materials are crucial to the function of living organisms, in the shells of molluscs1,2,3, the matrix of bone4, the teeth of sea urchins5, and the exoskeletons of coccoliths6. However, pathological biomineralization can be an undesirable crystallization process associated with human diseases7,8,9. The crystal growth of biogenic, natural and synthetic materials may be regulated by the action of modifiers, most commonly inhibitors, which range from small ions and molecules10,11 to large macromolecules12. Inhibitors adsorb on crystal surfaces and impede the addition of solute, thereby reducing the rate of growth13,14. Complex inhibitor–crystal interactions in biomineralization are often not well elucidated15. Here we show that two molecular inhibitors of calcium oxalate monohydrate crystallization—citrate and hydroxycitrate—exhibit a mechanism that differs from classical theory in that inhibitor adsorption on crystal surfaces induces dissolution of the crystal under specific conditions rather than a reduced rate of crystal growth. This phenomenon occurs even in supersaturated solutions where inhibitor concentration is three orders of magnitude less than that of the solute. The results of bulk crystallization, in situ atomic force microscopy, and density functional theory studies are qualitatively consistent with a hypothesis that inhibitor–crystal interactions impart localized strain to the crystal lattice and that oxalate and calcium ions are released into solution to alleviate this strain. Calcium oxalate monohydrate is the principal component of human kidney stones16,17,18,19 and citrate is an often-used therapy20, but hydroxycitrate is not. For hydroxycitrate to function as a kidney stone treatment, it must be excreted in urine. We report that hydroxycitrate ingested by non-stone-forming humans at an often-recommended dose leads to substantial urinary excretion. In vitro assays using human urine reveal that the molecular modifier hydroxycitrate is as effective an inhibitor of nucleation of calcium oxalate monohydrate nucleation as is citrate. Our findings support exploration of the clinical potential of hydroxycitrate as an alternative treatment to citrate for kidney stones.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Evans, J. S. “Tuning in” to mollusk shell nacre- and prismatic-associated protein terminal sequences. Implications for biomineralization and the construction of high performance inorganic-organic composites. Chem. Rev. 108, 4455–4462 (2008)
Falini, G., Albeck, S., Weiner, S. & Addadi, L. Control of aragonite or calcite polymorphism by mollusk shell macromolecules. Science 271, 67–69 (1996)
Meldrum, F. C. Calcium carbonate in biomineralisation and biomimetic chemistry. Int. Mater. Rev. 48, 187–224 (2003)
Dunlop, J. W. C. & Fratzl, P. Biological composites. Annu. Rev. Mater. Res. 40, 1–24 (2010)
Killian, C. E. et al. Mechanism of calcite co-orientation in the sea urchin tooth. J. Am. Chem. Soc. 131, 18404–18409 (2009)
Young, J. R., Didymus, J. M., Bown, P. R., Prins, B. & Mann, S. Crystal assembly and phylogenetic evolution in heterococcoliths. Nature 356, 516–518 (1992)
Rimer, J. D. et al. Crystal growth inhibitors for the prevention of L-cystine kidney stones through molecular design. Science 330, 337–341 (2010)
Olafson, K. N., Ketchum, M. A., Rimer, J. D. & Vekilov, P. G. Mechanisms of hematin crystallization and inhibition by the antimalarial drug chloroquine. Proc. Natl Acad. Sci. USA 112, 4946–4951 (2015)
Weissbuch, I. & Leiserowitz, L. Interplay between malaria, crystalline hemozoin formation, and antimalarial drug action and design. Chem. Rev. 108, 4899–4914 (2008)
Orme, C. A. et al. Formation of chiral morphologies through selective binding of amino acids to calcite surface steps. Nature 411, 775–779 (2001)
Davis, K. J., Dove, P. M. & De Yoreo, J. J. The role of Mg2+ as an impurity in calcite growth. Science 290, 1134–1137 (2000)
Graether, S. P. et al. β-helix structure and ice-binding properties of a hyperactive antifreeze protein from an insect. Nature 406, 325–328 (2000)
Sizemore, J. P. & Doherty, M. F. A new model for the effect of molecular imposters on the shape of faceted molecular crystals. Cryst. Growth Des. 9, 2637–2645 (2009)
Weissbuch, I., Addadi, L., Lahav, M. & Leiserowitz, L. Molecular recognition at crystal interfaces. Science 253, 637–645 (1991)
Dey, A., de With, G. & Sommerdijk, N. In situ techniques in biomimetic mineralization studies of calcium carbonate. Chem. Soc. Rev. 39, 397–409 (2010)
Coe, F. L., Parks, J. H. & Asplin, J. R. Medical process—the pathogenesis and treatment of kidney-stones. N. Engl. J. Med. 327, 1141–1152 (1992)
Wesson, J. A. & Ward, M. D. Pathological biomineralization of kidney stones. Elements 3, 415–421 (2007)
Nancollas, G. H. & Gardner, G. L. Kinetics of crystal growth of calcium oxalate monohydrate. J. Cryst. Growth 21, 267–276 (1974)
Ryall, R. L., Harnett, R. M. & Marshall, V. R. The effect of urine, pyrophosphate, citrate, magnesium and glycosaminoglycans on the growth and aggregation of calcium-oxalate crystals in vitro. Clin. Chim. Acta 112, 349–356 (1981)
Phillips, R. et al. Citrate salts for preventing and treating calcium containing kidney stones in adults. Cochrane Database Syst. Rev. 10, CD010057 (2015)
Qiu, S. R. et al. Molecular modulation of calcium oxalate crystallization by osteopontin and citrate. Proc. Natl Acad. Sci. USA 101, 1811–1815 (2004)
Farmanesh, S. et al. Specificity of growth inhibitors and their cooperative effects in calcium oxalate monohydrate crystallization. J. Am. Chem. Soc. 136, 367–376 (2014)
Wang, L. & Nancollas, G. H. Calcium orthophosphates: crystallization and dissolution. Chem. Rev. 108, 4628–4669 (2008)
Chernov, A. A. Formation of crystals in solutions. Contemp. Phys. 30, 251–276 (1989)
De Yoreo, J. J. & Vekilov, P. G. in Biomineralization Vol. 54 (eds Dove, P. M., De Yoreo, J. J. & Weiner, S. ) 57–93 (Mineralogical Society of America, 2003)
Lutsko, J. F. et al. Crystal growth cessation revisited: the physical basis of step pinning. Cryst. Growth Des. 14, 6129–6134 (2014)
Risti, R. I., Sherwood, J. N. & Wojciechowski, K. Assessment of the strain in small sodium-chlorate crystals and its relation to growth rate dispersion. J. Cryst. Growth 91, 163–168 (1988)
Ettinger, B. et al. Potassium-magnesium citrate is an effective prophylaxis against recurrent calcium oxalate nephrolithiasis. J. Urol. 158, 2069–2073 (1997)
Scales, C. D. J., Smith, A. C., Hanley, J. M. & Saigal, C. S. Prevalence of kidney stones in the United States. Eur. Urol. 62, 160–165 (2012)
Asplin, J. R. et al. Reduced crystallization inhibition by urine from men with nephrolithiasis. Kidney Int . 56, 1505–1516 (1999)
van Loon, L. J. C. et al. Effects of acute (-)-hydroxycitrate supplementation on substrate metabolism at rest and during exercise in humans. Am. J. Clin. Nutr. 72, 1445–1450 (2000)
Loe, Y. C. C., Bergeron, N., Rodriguez, N. & Schwarz, J. M. Gas chromatography/mass spectrometry method to quantify blood hydroxycitrate concentration. Anal. Biochem. 292, 148–154 (2001)
Parks, J. H., Worcester, E. M., Coe, F. L., Evan, A. P. & Lingeman, J. E. Clinical implications of abundant calcium phosphate in routinely analyzed kidney stones. Kidney Int . 66, 777–785 (2004)
Farmanesh, S. et al. High-throughput platform for design and screening of peptides as inhibitors of calcium oxalate monohydrate crystallization. J. Cryst. Growth 373, 13–19 (2013)
Tomazic, B. B. & Nancollas, G. H. A study of the phase-transformation of calcium oxalate trihydrate-monohydrate. Invest. Urol. 16, 329–335 (1979)
Lupulescu, A. I. & Rimer, J. D. In situ imaging of silicalite-1 surface growth reveals the mechanism of crystallization. Science 344, 729–732 (2014)
Farmanesh, S. et al. Natural promoters of calcium oxalate monohydrate crystallization. J. Am. Chem. Soc. 136, 12648–12657 (2014)
Jung, T. et al. Probing crystallization of calcium oxalate monohydrate and the role of macromolecule additives with in situ atomic force microscopy. Langmuir 20, 8587–8596 (2004)
Ahlrichs, R., Bar, M., Haser, M., Horn, H. & Kolmel, C. Electronic-structure calculations on workstation computers—the program system turbomole. Chem. Phys. Lett. 162, 165–169 (1989)
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098–3100 (1988)
Perdew, J. P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 33, 8822–8824 (1986)
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010)
Mpourmpakis, G., Caratzoulas, S. & Vlachos, D. G. What controls Au nanoparticle dispersity during growth? Nano Lett. 10, 3408–3413 (2010)
Feyereisen, M., Fitzgerald, G. & Komornicki, A. Use of approximate integrals in ab initio theory- An application in MP2 enegry calculations. Chem. Phys. Lett. 208, 359–363 (1993)
Weigend, F. & Haser, M. RI-MP2: first derivatives and global consistency. Theor. Chem. Acc. 97, 331–340 (1997)
Weigend, F., Haser, M., Patzelt, H. & Ahlrichs, R. RI-MP2: optimized auxiliary basis sets and demonstration of efficiency. Chem. Phys. Lett. 294, 143–152 (1998)
Klamt, A. & Schuurmann, G. COSMO—a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc., Perkin Trans. 2, 799–805 (1993)
Millan, A. Crystal growth shape of whewellite polymorphs: influence of structure distortions on crystal shape. Cryst. Growth Des. 1, 245–254 (2001)
Boll, M., Sorensen, E. & Balieu, E. Naturally occurring lactones and lactames. 3. Absolute configuration of hydroxycitric acid lactones—hibiscus acid and garcinia acid. Acta Chem. Scand. 23, 286 (1969)
Asplin, J. R. Evaluation of the kidney stone patient. Seminars Nephrol. 28, 99–110 (2008)
Farmanesh, S., Alamani, B. G. & Rimer, J. D. Identifying alkali metal inhibitors of crystal growth: a selection criterion based on ion pair hydration energy. Chem. Commun. 51, 13964–13967 (2015)
Martell, A. E. & Smith, R. M. in Critical Stability Constants Vol. 5, Ch. XI, 329–330 (Plenum Press, 1982)
ChemAxon. Hydroxycitric acid. http://www.chemicalize.org/structure/#!mol=hydroxycitrate&source=fp (2016)
Cabrera, N. & Vermileya, D. A. in Growth of Crystals from Solution (eds Doremus R. H. et al.) Ch. V, 393–410 (Wiley, 1958)
Voronkov, V. V. & Rashkovich, L. N. Step kinetics in the presence of mobile adsorbed impurity. J. Cryst. Growth 144, 107–115 (1994)
Burton, W. K., Cabrera, N. & Frank, F. C. The growth of crystals and the equilibrium structure of their surfaces. Phil. Trans. R. Soc. Lond. 243, 299–360 (1951)
Frank, F. C. The influence of dislocations on crystal growth. Discuss. Faraday Soc. 5, 48–54 (1949)
Lovette, M. A. et al. Crystal shape engineering. Ind. Eng. Chem. Res. 47, 9812–9833 (2008)
Snyder, R. C. & Doherty, M. F. Predicting crystal growth by spiral motion. Proc. R. Soc. A 465, 1145–1171 (2009)
Winn, D. & Doherty, M. F. Modeling crystal shapes of organic materials grown from solution. Am. Inst. Chem. Eng. J. 46, 1348–1367 (2000)
Linnikov, O. D. Investigation of the initial period of sulphate scale formation—Part 3. Variations of calcium sulphate crystal growth rates at its crystallization on a heat-exchange surface. Desalination 128, 47–55 (2000)
Malivuk, D. A., Zekic, A. A., Mitrovic, M. M. & Misailovic, B. M. Dissolution of sodium chlorate crystals in supersaturated solutions. J. Cryst. Growth 377, 164–169 (2013)
Cahn, J. W. & Larche, F. Surface stress and the chemical equilibrium of small crystals—II. Solid particles embedded in a solid matrix. Acta Metall . 30, 51–56 (1982)
van der Heijden, A. E. D. M. & van der Eerden, J. P. Growth rate dispersion: the role of lattice strain. J. Cryst. Growth 118, 14–26 (1992)
Wang, L. J. et al. Constant composition studies verify the utility of the cabrera-vermilyea (C-V) model in explaining mechanisms of calcium oxalate monohydrate crystallization. Cryst. Growth Des. 6, 1769–1775 (2006)
Acknowledgements
J.D.R. acknowledges support from the National Science Foundation (grant 1207441) and the Welch Foundation (grant E-1794). G.M. acknowledges start-up funds from the University of Pittsburgh and computational support from the Center for Simulation and Modeling, and the Extreme Science and Engineering Discovery Environment, which is supported by the National Science Foundation (grant ACI-1053575).
Author information
Authors and Affiliations
Contributions
J.C. performed data collection and analysis for bulk crystallization and in situ AFM studies, I.G. performed in vitro experiments in urine and analysed human trial samples, and M.G.T. performed DFT calculations. J.D.R. wrote the paper with help from G.M. and J.R.A., with all three authors contributing to the design and analysis of experiments. I.G. and M.G.T. contributed equally. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.D.R. and J.R.A. have filed a provisional patent application on the use of organic acids as growth inhibitors of pathological calcification.
Additional information
Reviewer Information Nature thanks J. Lieske, M. Sleutel and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 Examples of ISE measurements.
In situ ISE measurements of COM crystallization in the presence of CA (a) and HCA (b) at concentrations of 0 μg ml−1, 20 μg ml−1, 40 μg ml−1, 60 μg ml−1, 80 μg ml−1 and 100 μg ml−1. The y axis is the quantity of free calcium ions in the growth solution that are consumed during crystallization. Linear regression of each curve provides the rate of crystal growth. The percentage inhibition of COM crystallization is obtained by comparing the slopes of ISE curves in the presence of inhibitor (filled symbols) to the slope in the absence of inhibitor (open diamonds). ppm, parts per million.
Extended Data Figure 2 Inhibitor speciation and its effect on COM growth.
Here we compare calculations of inhibitor speciation with ISE measurements of COM percentage inhibition at pH 6.2 (solid bars) and pH 8.0 (patterned bars). a, Percentage of deprotonated CA and HCA species, calculated from equations (4)–(9). Fully dissociated species (charge −3) are represented by white bars and partially dissociated species (charge −2) are represented by grey bars. b, Results of ISE measurements in supersaturated calcium oxalate solution (S = 3.8) in the presence of CCA = 20 μg ml−1 (orange bars) and CHCA = 20 μg ml−1 (blue bars). The percentage inhibition of COM crystal growth slightly decreases at higher alkalinity. HCA is the more effective inhibitor irrespective of solution pH. Data are the average of more than 10 measurements (error bars are 1 s.d.; P < 0.05 comparing HCA to CA at both levels of pH).
Extended Data Figure 3 Optical micrographs of COM crystals.
Optical micrographs of COM crystals after heating a growth solution for 3 days at 60 °C. Here we compare the control sample in the absence of growth inhibitor (a) to solutions prepared with CCA = 20 μg ml−1 (b) and CHCA = 20 μg ml−1 (c). Scale bars, 100 μm.
Extended Data Figure 4 HCA-induced etch pits on the COM (100) surface.
a–c, Time-resolved images during in situ AFM measurements of a COM (100) crystal surface in the presence of HCA. The surface is first imaged in the absence of inhibitor (a) and then at CHCA = 0.25 μg ml−1 (b and c). The elapsed time between each deflection mode image is approximately 4 min. d–f, Height (or depth) profiles corresponding to the dashed lines in a–c, respectively. As shown in a and d, the COM (100) surface before the addition of HCA is comprised of single steps with height approximately 0.4 nm (see inset in d), which approximately corresponds to the unit cell dimension in the [100] direction (a = 0.6 nm)48. Depth profiles in e and f show the temporal evolution of a single etch pit. Quantitative analysis of etch pit dimensions with respect to depth d (g) and width w (h) reveal monotonic changes during 10 min of continuous AFM imaging. Schematics of an etch pit (left inset in g) with highlighted depth (right inset in g) and height (inset in h) are shown to aid visualization.
Extended Data Figure 5 Construction of Bliznakov plots.
a, Theoretical Bliznakov plot for crystal inhibitors that follow a step-pinning mode of action as a function of increasing calcium oxalate relative supersaturation σ (derived from ref. 65). b, Plots generated from in situ AFM data on COM (010) surfaces compare changes in step velocity in the [ ] direction (purple squares) and [021] direction (blue diamonds) as a function of increasing CHCA. The deviation of experimental data from theoretical trends suggests that step pinning is not the dominant mechanism by which HCA inhibits COM surface growth.
] direction (purple squares) and [021] direction (blue diamonds) as a function of increasing CHCA. The deviation of experimental data from theoretical trends suggests that step pinning is not the dominant mechanism by which HCA inhibits COM surface growth.
Extended Data Figure 6 Binding energy of inhibitors on the COM (100) surface.
The results of DFT calculations showing the adsorption configuration and binding energy of CA3− (a), CA2− (b), HCA3− (c) and Ox2− (d) on the COM (100) surface and of HCA3− (e) and CA3− (f) binding to a (001) step on the COM (100) surface. For these calculations, the surfaces are kept frozen (that is, unrelaxed). Atoms are coloured as follows: hydrogen (white), carbon (grey), oxygen (red) and calcium (green).
Extended Data Figure 7 CA and HCA interactions with relaxed COM surfaces.
Superimposed structures of CA and HCA interacting with unrelaxed (coloured balls and sticks) and partially relaxed (yellow sticks) surfaces of COM crystals. Side-view snapshots depict CA interaction with (100) (a) and (021) (b) surfaces and HCA interaction with (100) (c) and (021) (d) surfaces. Atoms are coloured as follows: hydrogen (white), carbon (grey), oxygen (red) and calcium (green). The (100) surface is practically unaffected by the presence of HCA, whereas the (021) surface shows dislocations due to strain induced by the high binding affinity of the inhibitor (see also Extended Data Table 1). The total energy of the (100) face changes by +18.8 kcal mol−1 owing to HCA adsorption compared to the +28.1 kcal mol−1 energy change of the (021) face (positive signs are endothermic and energy values correspond to the difference between single point energy calculations of the COM surface with inhibitors removed). The corresponding values for the total energy change of the (100) and (021) faces from the presence of the CA are +14.2 kcal mol−1 and +25.7 kcal mol−1, respectively. The partial relaxation of COM surfaces compared to unrelaxed surface calculations (Extended Data Fig. 6) does not alter the overall trend in inhibitor–crystal binding affinity.
Extended Data Figure 8 Complexation of organic acids with calcium.
DFT-calculated binding energy (scaled per number of molecules, N) for the complexation of organic anions HCA3−, CA2− and Ox2− with calcium ions. Note that the data for HCA3− and Ox2− are identical to Fig. 3g, and are merely placed here for direct comparison with CA2−. Dashed lines connecting symbols are added to guide the eye.
Supplementary information
COM (100) surface dissolution in the presence of CA
Time-elapsed sequence of AFM deflection mode images depicting the growth of hillocks on a COM (100) surface in supersaturated CaOx solution (S = 4.1). Continuous imaging is initially performed in the absence of CA (time t = 0 to 3.8 minutes) followed by the addition of the same growth solution containing CCA = 0.10 μg/mL. The formation of etch pits occurs almost instantaneously upon introducing the inhibitor. The total imaging time for the in situ AFM video is 14.4 minutes. (MOV 792 kb)
COM (100) surface dissolution in the presence of HCA
Time-elapsed sequence of AFM deflection mode images depicting the growth of hillocks on a COM (100) surface in supersaturated CaOx solution (S = 4.1). Continuous imaging is initially performed in the absence of HCA (time t = 0 to 6.7 minutes) followed by the addition of the same growth solution containing CHCA = 0.25 μg/mL. The formation of etch pits occurs almost instantaneously upon introducing the inhibitor. The total imaging time for the in situ AFM video is 32.7 minutes. (MOV 1030 kb)
COM (010) surface dissolution in the presence of HCA
Time-elapsed sequence of AFM deflection mode images depicting the growth of hillocks on a COM (010) surface in supersaturated CaOx solution (S = 4.1). Continuous imaging is initially performed in the absence of HCA (time t = 0 to 13.4 minutes) followed by the addition of the same growth solution containing CHCA = 0.10 μg/mL. The forward advancement of steps ceases upon introducing the inhibitor, and the steps recede (i.e., negative step velocity) toward the center of the screw dislocation with imaging time. Etch pit formation on terraces is observed during the course of surface dissolution at later times. The total imaging time for the in situ AFM video is 30.5 minutes. (MOV 1032 kb)
COM (010) surface dissolution in undersaturated solution
Time-elapsed sequence of AFM deflection mode images depicting the dissolution of hillocks on a COM (010) surface in undersaturated CaOx solution. Continuous imaging is initially performed in a supersaturated CaOx solution (S = 4.1) in the absence of inhibitor (not shown in the video). An undersaturated CaOx solution (S = 0.5) is introduced into the AFM liquid cell at time t = 0 minute. The forward advancement of steps ceases, and continuous imaging reveals that steps recede at a constant rate (i.e., negative step velocity) toward the center of the screw dislocation. Etch pit formation on terraces is also observed during the course of surface dissolution. The total imaging time for the in situ AFM video is 19.2 minutes. (MOV 464 kb)
Molecular conformations of inhibitor-calcium complexes
Animation of geometry optimization during DFT calculations of two HCA molecules and two CA molecules in their fully deprotonated state (charge = –3) that form complexes with three Ca2+ cations. The total system is neutral in these calculations. (MOV 6709 kb)
Rights and permissions
About this article
Cite this article
Chung, J., Granja, I., Taylor, M. et al. Molecular modifiers reveal a mechanism of pathological crystal growth inhibition. Nature 536, 446–450 (2016). https://doi.org/10.1038/nature19062
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature19062
This article is cited by
-
Mechanistic characterization of a Drosophila model of paraneoplastic nephrotic syndrome
Nature Communications (2024)
-
Boosting inhibition performance of natural polyphenols for the prevention of calcium oxalate kidney stones through synergistic cooperativity
Communications Materials (2023)
-
Nonclassical mechanisms to irreversibly suppress β-hematin crystal growth
Communications Biology (2023)
-
Oxalate homeostasis
Nature Reviews Nephrology (2023)
-
Growth strategy for solution-phase growth of two-dimensional nanomaterials via a unified model
Nature Synthesis (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.