Abstract
Zika virus (ZIKV) is a flavivirus that is responsible for the current epidemic in Brazil and the Americas1,2. ZIKV has been causally associated with fetal microcephaly, intrauterine growth restriction, and other birth defects in both humans3,4,5,6,7,8 and mice9,10,11. The rapid development of a safe and effective ZIKV vaccine is a global health priority1,2, but very little is currently known about ZIKV immunology and mechanisms of immune protection. Here we show that a single immunization with a plasmid DNA vaccine or a purified inactivated virus vaccine provides complete protection in susceptible mice against challenge with a strain of ZIKV involved in the outbreak in northeast Brazil. This ZIKV strain has recently been shown to cross the placenta and to induce fetal microcephaly and other congenital malformations in mice11. We produced DNA vaccines expressing ZIKV pre-membrane and envelope (prM-Env), as well as a series of deletion mutants. The prM-Env DNA vaccine, but not the deletion mutants, afforded complete protection against ZIKV, as measured by absence of detectable viraemia following challenge, and protective efficacy correlated with Env-specific antibody titers. Adoptive transfer of purified IgG from vaccinated mice conferred passive protection, and depletion of CD4 and CD8 T lymphocytes in vaccinated mice did not abrogate this protection. These data demonstrate that protection against ZIKV challenge can be achieved by single-shot subunit and inactivated virus vaccines in mice and that Env-specific antibody titers represent key immunologic correlates of protection. Our findings suggest that the development of a ZIKV vaccine for humans is likely to be achievable.
Similar content being viewed by others
Main
The World Health Organization declared the clusters of microcephaly and neurological disorders and their association with ZIKV infection to be a global public health emergency on February 1, 2016. ZIKV is believed to cause neuropathology in developing fetuses by crossing the placenta and targeting cortical neural progenitor cells9,10,11,12,13,14, leading to impaired neurogenesis and resulting in microcephaly and other congenital malformations. ZIKV has also been associated with neurologic conditions in adults, such as Guillain-Barré syndrome15.
Vaccines have been developed for other flaviviruses, including yellow fever virus, Japanese encephalitis virus, tick-borne encephalitis virus, and dengue viruses, but no vaccine currently exists for ZIKV. To develop preclinical challenge models for candidate ZIKV vaccines, we obtained low-passage ZIKV isolates from northeast Brazil (Brazil/ZKV2015; University of São Paulo)11 and Puerto Rico (PRVABC59; US Centers for Disease Control and Prevention) (Extended Data Fig. 1). We expanded these viruses in Vero cells to generate preclinical challenge stocks, which we termed ZIKV-BR and ZIKV-PR, respectively. These ZIKV strains are part of the Asian ZIKV lineage16 and differ from each other by five amino acids in the polyprotein (Extended Data Fig. 2). The Brazil/ZKV2015 strain has also recently been reported to recapitulate key clinical manifestations, including fetal microcephaly and intrauterine growth restriction, in wild-type SJL mice11. Similarly, the related French Polynesian H/PF/2013 strain has been shown to induce placental damage and fetal demise in Ifnar−/− C57BL/6 mice as well as in wild-type C57BL/6 mice following IFN-α receptor blockade10.
We designed ZIKV prM-Env immunogens based on the Brazil BeH815744 strain (Extended Data Fig. 2) and optimized them for increased antigen expression. We also designed deletion mutants lacking prM and/or lacking the transmembrane region (ΔTM) or the full stem (Δstem) of Env (Fig. 1a). Plasmid DNA vaccines encoding these antigens were produced, and transgene expression was verified by western blot (Fig. 1b). To assess the immunogenicity of these vaccines, groups of Balb/c mice (n = 5–10 per group) received a single immunization of 50 μg of each DNA vaccine by the intramuscular (i.m.) route at week 0. Env-specific antibody responses were evaluated at week 3 by ELISA. The prM-Env DNA vaccine elicited higher Env-specific antibody titers than did the Env DNA vaccine and all of the ΔTM and Δstem deletion mutants (Fig. 1c), indicating the importance of including prM as well as the full-length Env sequence. No prM-specific antibody responses were detected (Extended Data Fig. 3). The prM-Env DNA vaccine also induced ZIKV-specific neutralizing antibodies after a single immunization (Table 1), as measured by a virus-specific microneutralization assay17. In addition, the prM-Env DNA vaccine induced Env-specific CD8+ and CD4+ T-lymphocyte responses, as assessed by IFNγ ELISPOT and multiparameter intracellular cytokine staining assays (Fig. 1d, e).
a, Schema of ZIKV prM-Env immunogens and deletion mutants. b, Western blot of transgene expression from (1) prM-Env, (2) prM-Env(ΔTM), (3) prM-Env(Δstem), (4) Env, (5) Env(ΔTM), (6) Env(Δstem), and (7) sham DNA vaccines transfected in 293T cells. Balb/c mice (n = 5 per group) received a single immunization with 50 μg of these DNA vaccines by the i.m. route. c, Humoral immune responses were assessed at week 3 following vaccination by Env-specific ELISA. Red bars reflect medians. d, e, Cellular immune responses were assessed by IFNγ ELISPOT assays (d) and multi-parameter intracellular cytokine staining assays (e). Error bars reflect s.e.m.
To assess the protective efficacy of these DNA vaccines against ZIKV challenge, we infected vaccinated or sham control Balb/c mice at week 4 by the intravenous (i.v.) route with 105 viral particles (102 plaque-forming units (PFU)) of ZIKV-BR or ZIKV-PR. Viral loads following ZIKV challenge were quantitated by RT–PCR18. Sham-vaccinated mice inoculated with ZIKV-BR developed approximately 6 days of detectable viraemia with a mean peak viral load of 5.42 log copies per ml (range 4.55–6.57 log copies per ml; n = 10) on day 3 after challenge (Fig. 2a). In contrast, a single immunization with the prM-Env DNA vaccine provided complete protection against ZIKV-BR challenge with no detectable viraemia (<100 copies per ml) at any time point (n = 10). Complete protection was also observed when vaccinated mice were challenged at week 8 (data not shown). The prM-Env DNA vaccine also afforded complete protection against ZIKV-PR challenge (n = 5). ZIKV-PR replicated to slightly lower levels (mean peak viral load 4.96 log copies per ml; range 4.80–5.33 log copies per ml; n = 5) than did ZIKV-BR in sham controls. In contrast with the prM-Env DNA vaccine, the DNA vaccines lacking prM as well as the ΔTM and Δstem deletion mutants did not provide complete protection against ZIKV-BR challenge, although viral loads were still reduced in these animals as compared with sham controls (Fig. 2b).
a, Balb/c mice (n = 5 or 10 per group) received a single immunization by the i.m. route with 50 μg prM-Env DNA vaccine or a sham vaccine and were challenged at week 4 by the i.v. route with 105 viral particles (102 PFU) ZIKV-BR or ZIKV-PR. Serum viral loads are shown. b, Mice (n = 5 per group) received a single immunization with 50 μg of various DNA vaccines and were challenged with ZIKV-BR. c, d, Correlates of protective efficacy (c) and day 3 viral loads (d) are shown. Red bars reflect medians. P values and R2 values reflect t-tests and Spearman rank-correlation tests.
The varying degrees of protection obtained with this set of DNA vaccines allowed for an analysis of immune correlates of protection. Protective efficacy correlated with Env-specific binding antibody titers (P = 0.0005 comparing protected versus infected animals; Fig. 2c) as well as ZIKV-specific neutralizing antibody titers >10 (Table 1). In addition, peak viral loads on day 3 were inversely correlated with antibody titers (P < 0.0001, R2 = 0.72; Fig. 2d). These data suggest that Env-specific antibodies were critical for the protective efficacy of DNA vaccines against ZIKV-BR challenge. Mice that received two immunizations with the prM-Env DNA vaccine at week 0 and week 4 developed high neutralizing antibody titers of 1,022 at week 8 (Table 1) and were also protected against ZIKV-BR challenge (data not shown).
The prM-Env DNA vaccine also provided complete protection against ZIKV-BR challenge in SJL mice (Extended Data Fig. 4) and against both ZIKV-BR and ZIKV-PR challenge in C57BL/6 mice (Extended Data Figs 5 and 6). ZIKV-BR replicated efficiently in SJL mice, consistent with a previous study11, although at slightly lower levels (mean peak viral load 4.70 log copies per ml; range 3.50–5.92 log copies per ml; n = 5) than in Balb/c mice (Fig. 2a). In contrast, both ZIKV-BR and ZIKV-PR replicated poorly in C57BL/6 mice (Extended Data Fig. 5), also consistent with previous reports, potentially as a result of robust IFN-α-mediated innate immune restriction in this strain of mice10,11,19,20.
To investigate the immunological mechanism of protection against ZIKV-BR challenge, we purified IgG from serum from Balb/c mice vaccinated with prM-Env DNA. Passive infusion of varying quantities of purified IgG by the i.v. route resulted in median Env-specific log serum antibody titers of 2.82 (high), 2.35 (mid) and 1.87 (low) in recipient mice following adoptive transfer (Fig. 3a). All recipient mice with log serum antibody titers of 2.35 or higher were protected against ZIKV-BR challenge (Fig. 3b, c), demonstrating that protection can be mediated by vaccine-elicited IgG alone and confirming that the magnitude of Env-specific antibody titers correlates with protective efficacy (P < 0.0001, Fig. 3b). In contrast, only 1 out of 5 recipient mice that received low levels of Env-specific IgG were protected, although they still exhibited reduced viral loads compared with sham controls (Extended Data Fig. 7). These data define the minimum threshold of Env-specific antibody titers required for protection in this model.
a, Env-specific serum antibody titers in recipient Balb/c mice (n = 5 per group) following adoptive transfer of varying amounts (high, mid, low) of IgG purified from serum from mice vaccinated with prM-Env DNA or naive mice (sham). b, Correlates of protective efficacy. c, Serum viral loads in mice that received adoptive transfer of purified IgG from vaccinated mice and were challenged with ZIKV-BR. d, Serum viral loads in prM-Env-DNA-vaccinated mice that were depleted of CD4+ and/or CD8+ T lymphocytes before challenge with ZIKV-BR. Red bars reflect medians. P values reflect t-tests.
We next depleted CD4+ and/or CD8+ T lymphocytes in prM-Env-vaccinated mice on day −2 and day −1 before challenge (>99.9% efficiency; Extended Data Fig. 8). Depletion of these T-lymphocyte subsets did not detectably abrogate the protective efficacy of the prM-Env DNA vaccine against ZIKV-BR challenge (Fig. 3d). These data indicate that Env-specific T-lymphocyte responses were not required for protection in this model, although these findings do not exclude the possibility that ZIKV-specific cellular immune responses may be beneficial in other settings.
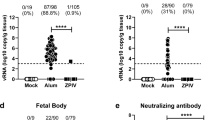
To extend these observations to a vaccine platform that has historically provided clinical efficacy against other flaviviruses, we explored the immunogenicity and protective efficacy of a ZIKV purified inactivated virus (PIV) vaccine derived from the Puerto Rico PRVABC59 strain. Groups of Balb/c mice (n = 5 per group) received a single immunization of 1 μg of the PIV vaccine with alum or alum alone by the i.m. or subcutaneous (s.c.) routes. Antibody titers were higher in the group that received the PIV vaccine by the i.m. route rather than by the s.c. route, as compared by ELISA (Fig. 4a). The PIV vaccine by both routes also induced ZIKV-specific neutralizing antibodies after a single immunization (Table 1). At week 4, all mice were i.v. challenged with ZIKV-BR as described above. Complete protection was observed in the group that received the PIV vaccine by the i.m. route (Fig. 4b, c). Two mice that received the PIV vaccine by the s.c. route showed brief low levels of viraemia (Fig. 4c), potentially consistent with the lower Env-specific binding antibody titers in this group (Fig. 4b).
Balb/c mice (n = 5 per group) received a single immunization by the i.m. or s.c. route with 1 μg PIV vaccine with alum, or alum alone, and were challenged at week 4 by the i.v. route with 105 viral particles (102 PFU) ZIKV-BR. a, Humoral immune responses were assessed at week 3 following vaccination by Env-specific ELISA. b, Correlates of protective efficacy. c, Serum viral loads are shown following ZIKV-BR challenge. Red bars reflect medians. P values reflect t-tests.
Our data demonstrate that a single immunization with a DNA vaccine or a PIV vaccine provided complete protection against parenteral ZIKV challenge in mice. The prM-Env DNA vaccine afforded protection in three strains of mice and against both ZIKV-BR and ZIKV-PR challenges, suggesting the generalizability of these observations. Protective efficacy was mediated by vaccine-elicited Env-specific antibodies, as evidenced by (1) statistical analyses of immune correlates of protection (Figs 2c, d), (2) adoptive transfer studies with purified IgG from vaccinated mice (Fig. 3a–c), and (3) T-lymphocyte depletion studies in vaccinated mice (Fig. 3d). The adoptive transfer studies also defined the threshold of Env-specific antibody titers required for protection in this model.
It is difficult to extrapolate directly the results from these vaccine studies in mice to potential clinical efficacy in humans. Nevertheless, the robust protection observed in the present studies and the clear immune correlates of protection suggest a path forward for ZIKV vaccine development in humans. Of note, similar antibody-based correlates of protection, including neutralizing antibody titers >10, have been reported for other flavivirus vaccines, including yellow fever virus, tick-borne encephalitis virus, and Japanese encephalitis virus21,22,23. Moreover, the ZIKV-BR challenge isolate used in the present study has been shown in wild-type SJL mice to recapitulate certain key clinical findings of ZIKV infection in humans, including fetal microcephaly and intrauterine growth retardation11. ZIKV-BR did not lead to a fatal outcome in wild-type Balb/c and SJL mice, as has been observed in Ifnar−/− C57BL/6 mice10,19,20, but the magnitude and duration of viraemia in Balb/c and SJL mice appear comparable with that in humans2, suggesting the potential relevance of this model. It is notable that ZIKV-BR replicated efficiently in wild-type Balb/c and SJL mice (Fig. 2a, Extended Data Fig. 4), but replicated poorly in wild-type C57BL/6 mice (Extended Data Fig. 5), which is consistent with previous observations10,11 and indicates important strain-specific differences for ZIKV infectivity. Further investigation into the immunologic mechanisms underlying these differences may lead to insights into innate immune control of ZIKV. Moreover, further characterization of the susceptible Balb/c and SJL murine models may facilitate future studies of ZIKV pathogenesis and the development of antiviral interventions. Future studies will also need to address the potential relevance of cross-reactive antibodies against dengue virus and other flaviviruses on ZIKV vaccine immunogenicity and protective efficacy.
The epidemiology of the current ZIKV outbreak1,2 and the clinical consequences for fetuses in pregnant women who become infected3,4,5,6,7,8 necessitate the urgent development of a ZIKV vaccine. Our data demonstrate that complete protection against ZIKV challenge was reliably and robustly achieved with both DNA vaccines and purified inactivated virus vaccines in susceptible mice. These vaccine platforms have previously been used at comparable doses to develop vaccines for other flaviviruses, including West Nile virus24,25, dengue viruses26,27, tick-borne encephalitis virus28,29, and Japanese encephalitis virus30, and may offer safety advantages over live attenuated and replicating flavivirus vaccines, particularly for pregnant women. Moreover, the magnitude of Env-specific antibody titers that provide complete protection against ZIKV challenge in mice should be readily achievable by DNA vaccines and purified inactivated virus vaccines in humans. Taken together, our findings provide substantial optimism that the development of a safe and effective ZIKV vaccine for humans will probably be feasible.
Methods
Animals
Balb/c, SJL, and C57BL/6 female mice at 6–8 weeks of age were purchased from Jackson Laboratories (Bar Harbour). Mice were vaccinated with 50 μg DNA vaccine in saline without adjuvant by the i.m. route or with 1 μg PIV vaccines with 100 μg alum (Alhydrogel; Brenntag Biosector, Denmark) adjuvant by the i.m. or s.c. routes in a 100 μl volume and were then challenged at week 4 by the i.v. route with 105 viral particles (102 plaque-forming units (PFU)) ZIKV-BR or ZIKV-PR. Animals were randomly allocated to groups. Immunologic and virologic assays were performed blinded. Sample size was determined to achieve 80% power to detect significant differences in protective efficacy. All animal studies were approved by the BIDMC Institutional Animal Care and Use Committee (IACUC).
DNA vaccines
ZIKV strain BeH815744 (accession number KU365780) was used to design transgenes, which were produced synthetically. Sequences were optimized for enhanced transgene expression. Pre-membrane and envelope (prM-Env; defined as amino acids 216–794 of the polyprotein) or Env alone were cloned into the mammalian expression plasmid pcDNA3.1+ (Invitrogen). Deletion mutants lacked the transmembrane (ΔTM) or stem (Δstem) regions of Env. A Kozak sequence and the Japanese encephalitis virus leader sequence were included24. Plasmids were produced with Machery-Nagel endotoxin-free gigaprep kits. Sequences were confirmed by double-stranded sequencing.
PIV vaccine
The ZIKV purified inactivated virus (PIV, also termed ZPIV) vaccine was produced at the Pilot Bioproduction Facility, Walter Reed Army Institute of Research, Silver Spring, MD, USA. The PIV vaccine was based on the Puerto Rican PRVABC59 isolate, which was obtained from the US Centers for Disease Control and Prevention, Fort Collins, CO, USA. The Vero cells used for passage and vaccine production were a derivative of a certified cell line manufactured at The Salk Institute, Swiftwater, PA. After inoculation, virus was collected on days 5 and 7, clarified by centrifugation and depth filter (0.45–0.2 μm), and treated with benzonase. The viral harvest was concentrated with an ultrafilter followed by purification using Captocore chromatography resin. The purified ZIKV was then inactivated with formalin (0.05%) at 22 °C for 7 days. Following inactivation, formalin was removed by dialysis, and the antigen concentration was adjusted. The final PIV vaccine was assessed for infectivity by passage in Vero cells followed by plaque assays to demonstrate inactivation.
ZIKV challenge stocks
ZIKV stocks were provided by University of São Paulo, Brazil (Brazil ZKV2015; ZIKV-BR11) and the US Centers for Disease Control and Prevention, USA (Puerto Rico PRVABC59; ZIKV-PR). Both strains were passage number 3. Low-passage-number Vero cells were then infected at a multiplicity of infection (MOI) of 0.01 PFU per cell. Supernatant was screened daily for viral titers and collected at peak growth. Culture supernatants were clarified by centrifugation, and fetal bovine serum was added to 20% final concentration (v/v) and stored at −80 °C. The concentration and infectivity of the stocks were determined by RT–PCR and PFU assays. The viral particle to PFU ratio of both stocks was approximately 1,000.
RT–PCR
Cap genes of available ZIKV genomes were aligned using Megalign (DNAstar), and primers and probes to a highly conserved region were designed using primer express v3.0 (Applied Biosystems). Primers were synthesized by Integrated DNA Technologies (Coralville) and probes by Biosearch Technologies (Petaluma). To assess viral loads, RNA was extracted from serum with a QIAcube HT (Qiagen). Reverse transcription and RT–PCR were performed as previously described18. The wild-type ZIKV BeH815744 Cap gene was used as a standard and was cloned into pcDNA3.1+ vector, and the AmpliCap-Max T7 High Yield Message Maker Kit was used to transcribe RNA (Cellscript). RNA was purified using the RNA clean and concentrator kit (Zymo Research), and RNA quality and concentration was assessed by the BIDMC Molecular Core Facility. Log dilutions of the RNA standard were reverse-transcribed and included with each RT–PCR assay. Viral loads were calculated as virus particles per ml. Assay sensitivity was 100 copies per ml. The infectivity of virus in peripheral blood from ZIKV challenged mice was confirmed by PFU assays.
PFU assay
Vero WHO cells were seeded in a MW6 plate to reach confluency at day 3. Cells were infected with log dilutions of ZIKV for 1 h and overlayed with agar. Cells were stained after 6 days of infection by neutral red staining. Plaques were counted, and titers were calculated by multiplying the number of plaques by the dilution and divided by the infection volume.
Western blot
To assess transgene expression from DNA vaccines, cell lysates obtained 48 h following lipofectamine 2000 (Invitrogen) transient transfection of 293T cells were mixed with reducing sample buffer, heated for 5 min at 100 °C, cooled on ice, and run on a precast 4–15% SDS–PAGE gel (Biorad). Protein was transferred to PVDF membranes using the iBlot dry blotting system (Invitrogen), and the membranes were blocked overnight at 4 °C in PBS-T (Dulbeco’s phosphate buffered saline + 0.2% V/V Tween 20 + 5% W/V non-fat milk powder). Following overnight blocking, the membranes were incubated for 1 h with PBS-T containing a 1:5,000 dilution of mouse anti-ZIKV Env monoclonal antibody (BioFront Technologies). Membranes were then washed 3 times with PBS-T and incubated for 1 h with PBS-T containing a 1:1,000 dilution of rabbit anti-mouse horseradish peroxidase (HRP) (Jackson ImmunoResearch). Membranes were then washed 3 times with PBS-T and developed using the Amersham ECL plus western blotting detection system (GE Healthcare).
ELISA
Mouse ZIKV Env ELISA kits (Alpha Diagnostic International) were used to determine endpoint antibody titers using a modified protocol. 96-well plates coated with ZIKV Env protein were first equilibrated at room temperature with 300 μl of kit working wash buffer for 5 min. 6 μl of mouse serum was added to the top row, and threefold serial dilutions were tested in the remaining rows. Samples were incubated at room temperature for 1 h, and plates washed 4 times. 100 μl of anti-mouse IgG HRP-conjugate working solution was then added to each well and incubated for 30 min at room temperature. Plates were washed 5 times, developed for 15 min at room temperature with 100 μl of 3,3′,5,5′–tetramehylbenzidine (TMB) substrate, and stopped by the addition of 100 μl of stop solution. Plates were analysed at 450 nm / 550 nm on a VersaMax microplate reader using Softmax Pro 6.0 software (Molecular Devices). ELISA endpoint titers were defined as the highest reciprocal serum dilution that yielded an absorbance >2-fold over background values.
Neutralization assay
A high-throughput ZIKV microneutralization (MN) assay was developed for measuring ZIKV-specific neutralizing antibodies as a modified version of a qualified dengue virus microneutralization assay used in clinical dengue vaccine trials17. Briefly, serum samples were serially diluted threefold in 96-well micro-plates, and 100 μl of ZIKV-PR containing 100 PFU were added to 100 μl of each serum dilution and incubated at 35 °C for 2 h. Supernatants were then transferred to microtiter plates containing confluent Vero cell monolayers (World Health Organization, NICSC-011038011038). After incubation for 4 d, cells were fixed with absolute ethanol: methanol for 1 h at −20 °C and washed three times with PBS. The pan-flavivirus monoclonal antibody 6B6-C1 conjugated to HRP (6B6-C1 was a gift from J. T. Roehrig, CDC) was then added to each well, incubated at 35 °C for 2 h, and washed with PBS. Plates were washed, developed with 3,3′,5,5′–tetramethylbenzidine (TMB) substrate for 50 min at room temperature, stopped with 1:25 phosphoric acid, and absorbance was read at 450 nm. For a valid assay, the average absorbance at 450 nm of three non-infected control wells had to be ≤ 0.5, and virus-only control wells had to be ≥ 0.9. Normalized absorbance values were calculated, and the MN50 titer was determined by a log mid-point linear regression model. The MN50 titer was calculated as the reciprocal of the serum dilution that neutralized ≥ 50% of ZIKV. Seropositivity was defined as a titer ≥ 1:10.
ELISPOT
ZIKV-specific cellular immune responses were assessed by IFNγ ELISPOT assays using pool of overlapping 15-amino-acid peptides covering the prM or Env proteins (JPT). 96-well multiscreen plates (Millipore) were coated overnight with 100 μl per well of 10 μg ml−1 anti-mouse IFNγ (BD Biosciences) in endotoxin-free Dulbecco’s PBS (D-PBS). The plates were then washed three times with D-PBS containing 0.25% Tween 20 (D-PBS-Tween), blocked for 2 h with D-PBS containing 5% FBS at 37 °C, washed three times with D-PBS-Tween, rinsed with RPMI 1640 containing 10% FBS to remove the Tween 20, and incubated with 2 μg ml−1 of each peptide and 5 × 105 mouse splenocytes in triplicate in 100 μl reaction mixture volumes. Following 18 h incubation at 37 °C, the plates were washed nine times with PBS-Tween and once with distilled water. The plates were then incubated with 2 μg ml−1 biotinylated anti-mouse IFNγ (BD Biosciences) for 2 h at room temperature, washed six times with PBS-Tween, and incubated for 2 h with a 1:500 dilution of streptavidin-alkaline phosphatase (Southern Biotechnology Associates). Following five washes with PBS-Tween and one with PBS, the plates were developed with nitroblue tetrazolium-5-bromo-4-chloro-3-indolyl-phosphate chromogen (Pierce), stopped by washing with tap water, air dried, and read using an ELISPOT reader (Cellular Technology Ltd). The numbers of spot-forming cells (SFC) per 106 cells were calculated. The medium background levels were typically <15 SFC per 106 cells.
Intracellular cytokine staining
ZIKV-specific CD4+ and CD8+ T-lymphocyte responses were assessed using splenocytes and analysed by flow cytometry. Cells were stimulated for 1 h at 37 °C with 2 μg ml−1 of overlapping 15-amino-acid peptides covering the prM or Env proteins (JPT). Following incubation, brefeldin-A and monensin (BioLegend) were added, and samples were incubated for 6 h at 37 °C. Cells were then washed, stained, permeabilized with Cytofix/Cytoperm (BD Biosciences). Data was acquired using an LSR II flow cytometer (BD Biosciences) and analysed using FlowJo v.9.8.3 (Treestar). Monoclonal antibodies included: CD4 (RM4-5), CD8α (53-6.7), CD44 (IM7), and IFNγ (XMG1.2). Antibodies were purchased from BD Biosciences, eBioscience, or BioLegend. Vital dye exclusion (LIVE/DEAD) was purchased from Life Technologies.
IgG purification and adoptive transfer
Serum was collected from prM-Env DNA-vaccinated mice or naive mice, and polyclonal IgG was purified using protein G purification kits (Thermo Fisher Scientific). Varying amounts of purified IgG was infused by the i.v. route into naive recipient mice before ZIKV challenge.
CD4+ and CD8+ T-lymphocyte depletion
Anti-CD4 (GK1.5) and/or anti-CD8 (2.43) (Bio X Cell) monoclonal antibodies were administered at doses of 500 μg per mouse to prM-Env DNA vaccinated mice by the i.p. route on day −2 and day −1 before ZIKV challenge. Antibody depletions were >99.9% efficient as determined by flow cytometry.
Statistical analyses
Analysis of virologic and immunologic data was performed using GraphPad Prism version 6.03 (GraphPad Software). Comparisons of groups was performed using t-tests and Wilcoxon rank-sum tests. Correlations were assessed by Spearman rank-correlation tests.
References
Fauci, A. S. & Morens, D. M. Zika virus in the Americas—yet another arbovirus threat. N. Engl. J. Med. 374, 601–604 (2016)
Petersen, L. R., Jamieson, D. J., Powers, A. M. & Honein, M. A. Zika virus. N. Engl. J. Med. 374, 1552–1563 (2016)
Mlakar, J. et al. Zika virus associated with microcephaly. N. Engl. J. Med. 374, 951–958 (2016)
Calvet, G. et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect. Dis. 16, 653–660 (2016)
Brasil, P. et al. Zika virus infection in pregnant women in Rio de Janeiro — preliminary report. N. Engl. J. Med. http://dx.doi.org/10.1056/NEJMoa1602412 (2016)
Driggers, R. W. et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N. Engl. J. Med. 374, 2142–2151 (2016)
Rasmussen, S. A., Jamieson, D. J., Honein, M. A. & Petersen, L. R. Zika virus and birth defects—reviewing the evidence for causality. N. Engl. J. Med. 374, 1981–1987 (2016)
Johansson, M. A., Mier-Y-Teran-Romero, L., Reefhuis, J., Gilboa, S. M. & Hills, S. L. Zika and the risk of microcephaly. N. Engl. J. Med. 375, 1–4 (2016)
Li, C. et al. Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell 19, 120–126 (2016)
Miner, J. J. et al. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 165, 1081–1091 (2016)
Cugola, F. R. et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534, 267–271 (2016)
Garcez, P. P. et al. Zika virus impairs growth in human neurospheres and brain organoids. Science 352, 816–818 (2016)
Qian, X. et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254 (2016)
Tang, H. et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell 18, 587–590 (2016)
Brasil, P. et al. Guillain-Barré syndrome associated with Zika virus infection. Lancet 387, 1482 (2016)
Faria, N. R. et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science 352, 345–349 (2016)
Thomas, S. J. et al. A phase II, randomized, safety and immunogenicity study of a re-derived, live-attenuated dengue virus vaccine in healthy adults. Am. J. Trop. Med. Hyg. 88, 73–88 (2013)
Tartaglia, L. J. et al. Production of mucosally transmissible SHIV challenge stocks from HIV-1 circulating recombinant form 01_AE env sequences. PLoS Pathog. 12, e1005431 (2016)
Lazear, H. M. et al. A mouse model of Zika virus pathogenesis. Cell Host Microbe 19, 720–730 (2016)
Rossi, S. L. et al. Characterization of a novel murine model to study Zika virus. Am. J. Trop. Med. Hyg. 94, 1362–1369 (2016)
Hombach, J., Solomon, T., Kurane, I., Jacobson, J. & Wood, D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine 23, 5205–5211 (2005)
Kreil, T. R., Burger, I., Bachmann, M., Fraiss, S. & Eibl, M. M. Antibodies protect mice against challenge with tick-borne encephalitis virus (TBEV)-infected macrophages. Clin. Exp. Immunol. 110, 358–361 (1997)
Mason, R. A., Tauraso, N. M., Spertzel, R. O. & Ginn, R. K. Yellow fever vaccine: direct challenge of monkeys given graded doses of 17D vaccine. Appl. Microbiol. 25, 539–544 (1973)
Martin, J. E. et al. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. J. Infect. Dis. 196, 1732–1740 (2007)
Ledgerwood, J. E. et al. A West Nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J. Infect. Dis. 203, 1396–1404 (2011)
Martinez, L. J. et al. Safety and immunogenicity of a dengue virus serotype-1 purified-inactivated vaccine: results of a phase 1 clinical trial. Am. J. Trop. Med. Hyg. 93, 454–460 (2015)
Fernandez, S. et al. An adjuvanted, tetravalent dengue virus purified inactivated vaccine candidate induces long-lasting and protective antibody responses against dengue challenge in rhesus macaques. Am. J. Trop. Med. Hyg. 92, 698–708 (2015)
Demicheli, V., Debalini, M. G. & Rivetti, A. Vaccines for preventing tick-borne encephalitis. Cochrane Database Syst. Rev. CD000977 (2009)
Demicheli, V., Graves, P., Pratt, M. & Jefferson, T. Vaccines for preventing tick-borne encephalitis. Cochrane Database Syst. Rev. CD000977 (2000)
Erra, E. O. & Kantele, A. The Vero cell-derived, inactivated, SA14-14-2 strain-based vaccine (Ixiaro) for prevention of Japanese encephalitis. Expert Rev. Vaccines 14, 1167–1179 (2015)
Acknowledgements
We thank P. Vasconcelos, K. Modjarrad, R. Olson, K. Kabra, C. Kannadka, C. Springer, G. Ballarini, G. Alter, K. Stephenson, F. Stephens, B. Lee, J. Jimenez, A. Chandrashekar, P. Penaloza and C. Cabral for generous advice, assistance and reagents. We acknowledge support from the Ragon Institute of MGH, MIT, and Harvard, the National Institutes of Health (AI095985, AI096040, AI100663, AI124377), and the São Paulo Research Foundation (FAPESP 2011/18703-2, 2014/17766-9). The views expressed in this manuscript are those of the authors and do not represent the official views of the Department of the Army or the Department of Defense.
Author information
Authors and Affiliations
Contributions
R.A.L., P.A. and D.H.B. designed the studies. J.P.S.P. and P.M.A.Z. developed the challenge virus. P.A., M.B., D.N., M.K., R.N., N.B.M. and Z.L. produced the DNA vaccines and conducted the virologic assays. R.A.B., R.G.J., K.H.E., N.L.M. and S.J.T. produced the PIV vaccines. R.A.L., M.J.I. and A.B.-Z. conducted the mouse studies. R.A.L., C.A.B., E.T.M., E.N.B., P.B.G., D.J., G.N., J.P.N., L.F.M., R.A.B. and R.G.J. conducted the immunologic assays. D.H.B. wrote the paper with all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information
Nature thanks A. Barrett and G. Screaton for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 ZIKV maximum likelihood phylogenetic tree.
The ZIKV-BR and ZIKV-PR challenge isolates are depicted with red arrows.
Extended Data Figure 2 ZIKV amino acid sequence comparisons.
Number of and percentage of amino acid differences in the polyprotein are shown for the following ZIKV isolates: Brazil/ZKV2015 (Brazil strain; ZIKV-BR challenge stock), PRVABC59 (Puerto Rico strain; ZIKV-PR challenge stock), BeH815744 (Brazil strain; immunogen design), H/PF/2013 (French Polynesian strain), and MR766 (African strain).
Extended Data Figure 3 prM-specific antibody responses in DNA-vaccinated mice.
In the experiment described in Fig. 2, humoral immune responses were assessed at week 3 following vaccination by prM-specific ELISA. Red bars reflect medians.
Extended Data Figure 4 Immunogenicity and protective efficacy of prM-Env DNA vaccine in SJL mice.
SJL mice (n = 5 per group) received a single immunization by the i.m. route with 50 μg prM-Env DNA vaccine or a sham vaccine and were challenged at week 4 by the i.v. route with 105 viral particles (102 PFU) ZIKV-BR. Humoral immune responses were assessed at week 3 after vaccination by Env-specific ELISA (top). Red bars reflect medians. Serum viral loads are shown following ZIKV-BR challenge (bottom).
Extended Data Figure 5 Protective efficacy of prM-Env DNA vaccine in C57BL/6 mice.
C57BL/6 mice (n = 5 per group) received a single immunization by the i.m. route with 50 μg prM-Env DNA vaccine or a sham vaccine and were challenged at week 4 by the i.v. route with 105 viral particles (102 PFU) ZIKV-BR or ZIKV-PR. Serum viral loads are shown following challenge.
Extended Data Figure 6 Protective efficacy of various DNA vaccines in C57BL/6 mice.
C57BL/6 mice (n = 5 per group) received a single immunization by the i.m. route with 50 μg of various DNA vaccines and were challenged at week 4 by the i.v. route with 105 viral particles (102 PFU) ZIKV-BR. Serum viral loads are shown following challenge.
Extended Data Figure 7 Adoptive transfer of low titers of Env-specific IgG.
Serum viral loads in mice that received adoptive transfer of low titers of Env-specific IgG (as defined in Fig. 3a) and were then challenged with ZIKV-BR.
Extended Data Figure 8 CD4+ and CD8+ T-lymphocyte depletion.
CD4+ and/or CD8+ T-lymphocyte depletion following monoclonal antibody treatment of Balb/c mice vaccinated with prM-Env DNA.
Rights and permissions
About this article
Cite this article
Larocca, R., Abbink, P., Peron, J. et al. Vaccine protection against Zika virus from Brazil. Nature 536, 474–478 (2016). https://doi.org/10.1038/nature18952
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature18952
This article is cited by
-
Flavivirus prM interacts with MDA5 and MAVS to inhibit RLR antiviral signaling
Cell & Bioscience (2023)
-
Immunization against Zika by entrapping live virus in a subcutaneous self-adjuvanting hydrogel
Nature Biomedical Engineering (2023)
-
A hierarchical intervention scheme based on epidemic severity in a community network
Journal of Mathematical Biology (2023)
-
Neurocognitive impact of Zika virus infection in adult rhesus macaques
Journal of Neuroinflammation (2022)
-
ZIKV-envelope proteins induce specific humoral and cellular immunity in distinct mice strains
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.