Abstract
Nitrogen oxides are essential for the formation of secondary atmospheric aerosols and of atmospheric oxidants such as ozone and the hydroxyl radical, which controls the self-cleansing capacity of the atmosphere1. Nitric acid, a major oxidation product of nitrogen oxides, has traditionally been considered to be a permanent sink of nitrogen oxides1. However, model studies predict higher ratios of nitric acid to nitrogen oxides in the troposphere than are observed2,3. A ‘renoxification’ process that recycles nitric acid into nitrogen oxides has been proposed to reconcile observations with model studies2,3,4, but the mechanisms responsible for this process remain uncertain5,6,7,8,9. Here we present data from an aircraft measurement campaign over the North Atlantic Ocean and find evidence for rapid recycling of nitric acid to nitrous acid and nitrogen oxides in the clean marine boundary layer via particulate nitrate photolysis. Laboratory experiments further demonstrate the photolysis of particulate nitrate collected on filters at a rate more than two orders of magnitude greater than that of gaseous nitric acid, with nitrous acid as the main product. Box model calculations based on the Master Chemical Mechanism10,11 suggest that particulate nitrate photolysis mainly sustains the observed levels of nitrous acid and nitrogen oxides at midday under typical marine boundary layer conditions. Given that oceans account for more than 70 per cent of Earth’s surface, we propose that particulate nitrate photolysis could be a substantial tropospheric nitrogen oxide source. Recycling of nitrogen oxides in remote oceanic regions with minimal direct nitrogen oxide emissions could increase the formation of tropospheric oxidants and secondary atmospheric aerosols on a global scale.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Finlayson-Pitts, B. J. & Pitts, J. N., Jr. Chemistry of the Upper and Lower Atmosphere: Theory, Experiments and Applications (Academic, 2000).
Wang, K., Zhang, Y., Nenes, A. & Fountoukis, C. Implementation of dust emission and chemistry into the Community Multiscale Air Quality modeling system and initial application to an Asian dust storm episode. Atmos. Chem. Phys. 12, 10209–10237 (2012).
Deng, J., Wang, T., Liu, L. & Jiang, F. Modeling heterogeneous chemical processes on aerosol surface. Particuology 8, 308–318 (2010).
Kumar, R. et al. Effects of dust aerosols on tropospheric chemistry during a typical pre-monsoon season dust storm in northern India. Atmos. Chem. Phys. 14, 6813–6834 (2014).
Zhou, X. et al. Nitric acid photolysis on surfaces in low-NO x environments: significant atmospheric implications. Geophys. Res. Lett. 30, 2217 (2003).
Baergen, A. M. & Donaldson, D. J. Photochemical renoxification of nitric acid on real urban grime. Environ. Sci. Technol. 47, 815–820 (2013).
Zhu, C., Xiang, B., Chu, L. T. & Zhu, L. 308 nm photolysis of nitric acid in the gas phase, on aluminum surfaces, and on ice films. J. Phys. Chem. A 114, 2561–2568 (2010).
Du, J. & Zhu, L. Quantification of the absorption cross sections of surface-adsorbed nitric acid in the 335–365 nm region by Brewster angle cavity ring-down spectroscopy. Chem. Phys. Lett. 511, 213–218 (2011).
Zhou, X. et al. Nitric acid photolysis on forest canopy surface as a source for tropospheric nitrous acid. Nature Geosci. 4, 440–443 (2011).
Saunders, S. M., Jenkin, M. E., Derwent, R. G. & Pilling, M. J. Protocol for the development of the Master Chemical Mechanism, MCM v3 (part A): tropospheric degradation of non-aromatic volatile organic compounds. Atmos. Chem. Phys. 3, 161–180 (2003).
Jenkin, M. E., Saunders, S. M., Wagner, V. & Pilling, M. J. Protocol for the development of the Master Chemical Mechanism, MCM v3 (part B): tropospheric degradation of aromatic volatile organic compounds. Atmos. Chem. Phys. 3, 181–193 (2003).
Ren, X. et al. OH, HO2, and OH reactivity during the PMTACS–NY Whiteface Mountain 2002 campaign: observations and model comparison. J. Geophys. Res. 111, D10S03 (2006).
Czader, B. H. et al. Modeling nitrous acid and its impact on ozone and hydroxyl radical during the Texas Air Quality Study 2006. Atmos. Chem. Phys. 12, 6939–6951 (2012).
Lee, J. D. et al. Year-round measurements of nitrogen oxides and ozone in the tropical North Atlantic marine boundary layer. J. Geophys. Res. 114, D21302 (2009).
Zhang, N. et al. Aircraft measurement of HONO vertical profiles over a forested region. Geophys. Res. Lett. 36, L15820 (2009).
Li, X. et al. Missing gas-phase source of HONO inferred from Zeppelin measurements in the troposphere. Science 344, 292–296 (2014).
Kleffmann, J. Daytime sources of nitrous acid (HONO) in the atmospheric boundary layer. ChemPhysChem 8, 1137–1144 (2007).
Jankowski, J. J., Kieber, D. J., Mopper, K. & Neale, P. J. Development and intercalibration of ultraviolet solar actinometers. Photochem. Photobiol. 71, 431–440 (2000).
Ramazan, K. A., Syomin, D. & Finlayson-Pitts, B. J. The photochemical production of HONO during the heterogeneous hydrolysis of NO2 . Phys. Chem. Chem. Phys. 6, 3836–3843 (2004).
Turekian, V. C., Macko, S. A. & Keene, W. C. Concentrations, isotopic compositions, and sources of size-resolved, particulate organic carbon and oxalate in near-surface marine air at Bermuda during spring. J. Geophys. Res. 108 (D5), 4157 (2003).
Nissenson, P., Knox, C. J. H., Finlayson-Pitts, B. J., Philips, L. F. & Dabdub, D. Enhanced photolysis in aerosols: evidence for important surface effects. Phys. Chem. Chem. Phys. 8, 4700–4710 (2006).
Richards, N. K. et al. Nitrate ion photolysis in thin water films in the presence of bromide ions. J. Phys. Chem. A 115, 5810–5821 (2011).
Zhou, X. et al. Snowpack photochemical production of HONO: a major source of OH in the Arctic boundary layer in springtime. Geophys. Res. Lett. 28, 4087–4090 (2001).
Val Martin, M., Honrath, R. E., Owen, R. C. & Li, Q. B. Seasonal variation of nitrogen oxides in the central North Atlantic lower free troposphere. J. Geophys. Res. 113, D17307 (2008).
Helas, G. & Warneck, P. Background NO x mixing ratios in air masses over the North Atlantic Ocean. J. Geophys. Res. 86 (C8), 7283–7290 (1981).
Li, S., Matthews, J. & Sinha, A. Atmospheric hydroxyl radical production from electronically excited NO2 and H2O. Science 319, 1657–1660 (2008).
Carr, S., Heard, D. E. & Blitz, M. A. Comment on “Atmospheric hydroxyl radical production from electronically excited NO2 and H2O”. Science 324, 336b (2009).
Ye, C. et al. Comment on “Missing gas-phase source of HONO inferred from Zeppelin measurements in the troposphere”. Science 326, 1657–1659 (2015).
Savarino, J. et al. Isotopic composition of atmospheric nitrate in a tropical marine boundary layer. Proc. Natl Acad. Sci. USA 110, 17668–17673 (2013).
Browne, E. C. et al. Observations of total RONO2 over the boreal forest: NO x sinks and HNO3 sources. Atmos. Chem. Phys. 13, 4543–4562 (2013).
Zhang, N. et al. Measurements of ambient HONO concentrations and vertical HONO flux above a northern Michigan forest canopy. Atmos. Chem. Phys. 12, 8285–8296 (2012).
Huang, G., Zhou, X., Deng, G., Qiao, H. & Civerolo, K. Measurements of atmospheric nitrous acid and nitric acid. Atmos. Environ. 36, 2225–2235 (2002).
Ridley, B. et al. Florida thunderstorms: a faucet of reactive nitrogen to the upper troposphere. J. Geophys. Res. 109, D17305 (2004).
Mauldin, R. et al. South Pole Antarctica observations and modeling results: new insights on HOx radical and sulfur chemistry. Atmos. Environ. 44, 572–581 (2010).
Hornbrook, R. S. et al. Measurements of tropospheric HO2 and RO2 by oxygen dilution modulation and chemical ionization mass spectrometry. Atmos. Meas. Tech. 4, 735–756 (2011).
Platt, U. & Stutz, J. Differential Optical Absorption Spectroscopy: Principles and Applications (Springer, 2008).
Shetter, R. E., Cinquini, L., Lefer, B. L., Hall, S. R. & Madronich, S. Comparison of airborne measured and calculated spectral actinic flux and derived photolysis frequencies during the PEM Tropics B mission. J. Geophys. Res. 108 (D2), 8234 (2003).
Flagan, R. C. Electrical mobility methods for sub-micrometer particle characterization. In Aerosol Measurement: Principles, Techniques, and Applications 3rd edn (eds Kulkarni, P. Baron, P. A. & Willeke, K. ), 339–364 (John Wiley & Sons, 2011).
de Gouw, J. & Warneke, C. Measurements of volatile organic compounds in the Earth’s atmosphere using proton-transfer-reaction mass spectrometry. Mass Spectrom. Rev. 26, 223–257 (2007).
Hornbrook, R. S. et al. Observations of nonmethane organic compounds during ARCTAS—part 1: Biomass burning emissions and plume enhancements. Atmos. Chem. Phys. 11, 11103–11130 (2011).
Gierczak, T., Jimenez, E., Riffault, V., Burkholder, J. B. & Ravishankara, A. R. Thermal decomposition of HO2NO2 (peroxynitric acid, PNA): rate coefficient and determination of the enthalpy of formation. J. Phys. Chem. A 109, 586–596 (2005).
Cantrell, C. A. et al. Steady state free radical budgets and ozone photochemistry during TOPSE. J. Geophys. Res. 108 (D4), 8361 (2003).
Stohl, A., Forster, C., Frank, A., Seibert, P. & Wotawa, G. The Lagrangian particle dispersion model FLEXPART version 6.2. Atmos. Chem. Phys. 5, 2461–2474 (2005).
Stohl, et al. A replacement for simple back trajectory calculations in the interpretation of atmospheric trace substance measurements. Atmos. Environ. 36, 4635–4648 (2002).
Zhang, N. Distributions and Sources of HONO in the Rural Troposphere. PhD thesis, http://gradworks.umi.com/34/89/3489695.html (State Univ. New York, 2011).
Sander, S. et al. Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies, Evaluation No. 17 JPL Publication 10-6, http://jpldataeval.jpl.nasa.gov/pdf/JPL09_16Nov09_Sander.pdf (Jet Propulsion Laboratory, 2011).
Weis, D. D. & Ewing, G. E. The reaction of nitrogen dioxide with sea salt aerosol. J. Phys. Chem. A 103, 4865–4873 (1999).
Joseph, D. M., Ashworth, S. H. & Plane, J. M. C. On the photochemistry of IONO2: absorption cross section (240–370 nm) and photolysis product yields at 248 nm. Phys. Chem. Chem. Phys. 9, 5599–5607 (2007).
McFiggans, G. et al. A modeling study of iodine chemistry in the marine boundary layer. J. Geophys. Res. 105 (D11), 14371–14385 (2000).
Dix, B. et al. Detection of iodine monoxide in the tropical free troposphere. Proc. Natl Acad. Sci. USA 110, 2035–2040 (2013).
Zhang, J., Dransfield, T. & Donahue, N. M. On the mechanism for nitrate formation via the peroxy radical + NO reaction. J. Phys. Chem. A 108, 9082–9095 (2004).
Acknowledgements
This research is funded by National Science Foundation (NSF) grants (AGS-1216166, AGS-1215712, and AGS-1216743). We would like to acknowledge operational, technical and scientific support provided by NCAR’s Earth Observing Laboratory, sponsored by the National Science Foundation. Any opinions, findings, conclusions or recommendations expressed in this paper are those of the authors and do not necessarily reflect the views of NSF.
Author information
Authors and Affiliations
Contributions
Ye, C. and Zhou, X. designed and performed the field and laboratory studies, interpreted the data and write the manuscript with inputs from all the co-authors; Cantrell, C. and Ye, C. performed model simulations.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
The data are available in our project data archive (http://data.eol.ucar.edu/master_list/?project=SAS).
Extended data figures and tables
Extended Data Figure 1 Typical back-trajectories of air masses in the MBL.
a, 5 July 2013; b, 8 July 2013. The air mass circulated within the North Atlantic Ocean under a Bermuda high-pressure system for several days before reaching the measurement locations. On 8 July 2013, the air mass near the coast was also occasionally affected slightly by fresh emissions from the southeast coast of the USA. See ref. 44 for definition of the cluster centroid. The C-130 flight tracks are shown in upper panels by the lines from 33° 39′ N, 77° 42′ W to 32° 11′ N, 77° 41′ W over the North Atlantic Ocean. The altitudes of cluster centroids in the lower panels are in metres above ground level (AGL).
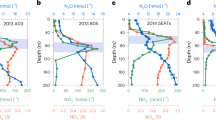
Extended Data Figure 2 Typical diurnal HONO and NOx budgets in the clean MBL calculated by the MCM v3.2.
a, HONO budget; b, NOx budget. ‘HONO photolysis’ and ‘HONO+OH’ represent HONO sinks contributed by HONO photolysis and the gas-phase reactions of HONO with the OH radicals. ‘Nitrate photolysis’, ‘NO+OH’, ‘Excited NO2’, ‘HO2·H2O+NO2’ and ‘NO2 heterogeneous’ represent HONO sources contributed by pNO3 photolysis, the gas-phase reactions of NO with OH, of excited NO2 with H2O and of HO2·H2O with NO2, and the heterogeneous reactions of NO2 on sea-salt aerosol particles, respectively. ‘HOx+NOx’ is the NOx sink contributed by gas-phase reactions of NO2 with OH and of NO with HO2 with a branching ratio of 0.5% (ref. 46). ‘Halogen nitrate’ is the NOx sink contributed by gas-phase reactions of NO2 with primarily BrO and IO (ref. 29). ‘Organic nitrate’ is the NOx sink contributed by reactions of RO2 radicals with NO with an effective branching ratio of 7% (refs 30 and 51). ‘Other sinks’ represents other minor NOx sinks, such as hydrolysis of N2O5. ‘Nitrate photolysis’ is the NOx source contributed by pNO3 photolysis and ‘Other sources’ represents other minor NOx sources, such as photolysis of gaseous HNO3.
Extended Data Figure 3 HONO observation comparison between the UCLA Mini-DOAS instrument and the LPAP instrument within two plumes during the research flight on 20 June 2013.
The Mini-DOAS instrument is a limb-scanning Max-DOAS instrument, and the analysis incorporates the DOAS approach36. The differential slant column densities of HONO were scaled to the differential slant column density analysis of HCHO, which were retrieved in the same spectral interval and multiplied by the in situ HCHO measurements, provided by the TOGA instrument40 to derive HONO mixing ratios. The Mini-DOAS data was obtained along various elevation viewing angles from +45° to −10°. Because of the position of the aircraft relative to the plumes, the plume geometry, and the HCHO scaling, the derived HONO mixing ratios do not depend on elevation viewing angle and this information was therefore omitted from the figure. Error bars (±1 s.d.) accompany each DOAS measurement. DOAS measurements influenced by clouds or aircraft manoeuvres have been removed from the figure. For better visibility negative DOAS values, which were statistically indistinguishable from zero, were set to 0 p.p.t.v. and the error bar was removed. Red error bars show a 20% uncertainty (±1 s.d.) for LPAP HONO results.
Extended Data Figure 4 Comparison of the xenon arc lamp light spectrum with the solar actinic spectrum.
The xenon light source was filtered by a 4-mm 7740 Pyrex filter, and the solar actinic flux was the 10-min measurement data at 360 m above sea level from 18:55 to 19:05 utc during RF14.
Extended Data Figure 5 Typical diurnal profiles of HO2+RO2 and OH radicals in the clean MBL as observed and calculated by the MCM v3.2.
a, HO2+RO2 radicals; b, OH radicals. The calculated (Model) and measured (Obs) ratio of HO2 radicals to RO2 radicals (CH3O2 plus higher RO2) is about 1. The error bars represent ±1 s.d. of the model calculations and observations.
Rights and permissions
About this article
Cite this article
Ye, C., Zhou, X., Pu, D. et al. Rapid cycling of reactive nitrogen in the marine boundary layer. Nature 532, 489–491 (2016). https://doi.org/10.1038/nature17195
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature17195
This article is cited by
-
Elucidating HONO formation mechanism and its essential contribution to OH during haze events
npj Climate and Atmospheric Science (2023)
-
Synthesizing evidence for the external cycling of NOx in high- to low-NOx atmospheres
Nature Communications (2023)
-
Important contributions of non-fossil fuel nitrogen oxides emissions
Nature Communications (2021)
-
Capillary shrinkage of graphene oxide hydrogels
Science China Materials (2020)
-
Matrix effect on surface-catalyzed photolysis of nitric acid
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.