Abstract
Directional control of tip-growing cells is essential for proper tissue organization and cell-to-cell communication in animals and plants1,2. In the sexual reproduction of flowering plants, the tip growth of the male gametophyte, the pollen tube, is precisely guided by female cues to achieve fertilization3. Several female-secreted peptides have recently been identified as species-specific attractants that directly control the direction of pollen tube growth4,5,6. However, the method by which pollen tubes precisely and promptly respond to the guidance signal from their own species is unknown. Here we show that tip-localized pollen-specific receptor-like kinase 6 (PRK6) with an extracellular leucine-rich repeat domain is an essential receptor for sensing of the LURE1 attractant peptide in Arabidopsis thaliana under semi-in-vivo conditions, and is important for ovule targeting in the pistil. PRK6 interacted with pollen-expressed ROPGEFs (Rho of plant guanine nucleotide-exchange factors), which are important for pollen tube growth through activation of the signalling switch Rho GTPase ROP1 (refs 7, 8). PRK6 conferred responsiveness to AtLURE1 in pollen tubes of the related species Capsella rubella. Furthermore, our genetic and physiological data suggest that PRK6 signalling through ROPGEFs and sensing of AtLURE1 are achieved in cooperation with the other PRK family receptors, PRK1, PRK3 and PRK8. Notably, the tip-focused PRK6 accumulated asymmetrically towards an external AtLURE1 source before reorientation of pollen tube tip growth. These results demonstrate that PRK6 acts as a key membrane receptor for external AtLURE1 attractants, and recruits the core tip-growth machinery, including ROP signalling proteins. This work provides insights into the orchestration of efficient pollen tube growth and species-specific pollen tube attraction by multiple receptors during male–female communication.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Itofusa, R. & Kamiguchi, H. Polarizing membrane dynamics and adhesion for growth cone navigation. Mol. Cell. Neurosci. 48, 332–338 (2011)
Yang, Z. Cell polarity signaling in Arabidopsis. Annu. Rev. Cell Dev. Biol. 24, 551–575 (2008)
Higashiyama, T. & Takeuchi, H. The mechanism and key molecules involved in pollen tube guidance. Annu. Rev. Plant Biol. 66, 393–413 (2015)
Okuda, S. et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature 458, 357–361 (2009)
Márton, M. L., Fastner, A., Uebler, S. & Dresselhaus, T. Overcoming hybridization barriers by the secretion of the maize pollen tube attractant ZmEA1 from Arabidopsis ovules. Curr. Biol. 22, 1194–1198 (2012)
Takeuchi, H. & Higashiyama, T. A species-specific cluster of defensin-like genes encodes diffusible pollen tube attractants in Arabidopsis. PLoS Biol. 10, e1001449 (2012)
Kaothien, P. et al. Kinase partner protein interacts with the LePRK1 and LePRK2 receptor kinases and plays a role in polarized pollen tube growth. Plant J. 42, 492–503 (2005)
Zhang, Y. & McCormick, S. A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase Rop in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 104, 18830–18835 (2007)
Higashiyama, T. et al. Pollen tube attraction by the synergid cell. Science 293, 1480–1483 (2001)
Jones-Rhoades, M. W., Borevitz, J. O. & Preuss, D. Genome-wide expression profiling of the Arabidopsis female gametophyte identifies families of small, secreted proteins. PLoS Genet. 3, e171 (2007)
Shiu, S. H. & Bleecker, A. B. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Natl Acad. Sci. USA 98, 10763–10768 (2001)
Palanivelu, R. & Preuss, D. Distinct short-range ovule signals attract or repel Arabidopsis thaliana pollen tubes in vitro. BMC Plant Biol. 6, 7 (2006)
Qin, Y. et al. Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genet. 5, e1000621 (2009)
Chang, F., Gu, Y., Ma, H. & Yang, Z. AtPRK2 promotes ROP1 activation via RopGEFs in the control of polarized pollen tube growth. Mol. Plant 6, 1187–1201 (2013)
Wengier, D. et al. The receptor kinases LePRK1 and LePRK2 associate in pollen and when expressed in yeast, but dissociate in the presence of style extract. Proc. Natl Acad. Sci. USA 100, 6860–6865 (2003)
Tang, W., Ezcurra, I., Muschietti, J. & McCormick, S. A cysteine-rich extracellular protein, LAT52, interacts with the extracellular domain of the pollen receptor kinase LePRK2. Plant Cell 14, 2277–2287 (2002)
Tang, W., Kelley, D., Ezcurra, I., Cotter, R. & McCormick, S. LeSTIG1, an extracellular binding partner for the pollen receptor kinases LePRK1 and LePRK2, promotes pollen tube growth in vitro. Plant J. 39, 343–353 (2004)
Lu, Y. et al. Pollen tubes lacking a pair of K+ transporters fail to target ovules in Arabidopsis. Plant Cell 23, 81–93 (2011)
Berken, A. Thomas. C. & Wittinghofer. A. A new family of RhoGEFs activates the Rop molecular switch in plants. Nature 436, 1176–1180 (2005)
Oda, Y. & Fukuda, H. Initiation of cell wall pattern by a Rho- and microtubule-driven symmetry breaking. Science 337, 1333–1336 (2012)
Li, H., Lin, Y., Heath, R. M., Zhu, M. X. & Yang, Z. Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell 11, 1731–1742 (1999)
Gu, Y. et al. A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J. Cell Biol. 169, 127–138 (2005)
Liu, J. et al. Membrane-bound RLCKs LIP1 and LIP2 are essential male factors controlling male-female attraction in Arabidopsis. Curr. Biol. 23, 993–998 (2013)
Silverstein, K. A. et al. Small cysteine-rich peptides resembling antimicrobial peptides have been under-predicted in plants. Plant J. 51, 262–280 (2007)
Lee, J. S. et al. Direct interaction of ligand-receptor pairs specifying stomatal patterning. Genes Dev. 26, 126–136 (2012)
Lee, J. S. et al. Competitive binding of antagonistic peptides fine-tunes stomatal patterning. Nature 522, 439–443 (2015)
Haruta, M., Sabat, G., Stecker, K., Minkoff, B. B. & Sussman, M. R. A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343, 408–411 (2014)
Takayama, S. et al. Direct ligand-receptor complex interaction controls Brassica self-incompatibility. Nature 413, 534–538 (2001)
Slotte, T. et al. The Capsella rubella genome and the genomic consequences of rapid mating system evolution. Nature Genet. 45, 831–835 (2013)
Winter, D. et al. An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2, e718 (2007)
Hamamura, Y. et al. Live imaging of calcium spikes during double fertilization in Arabidopsis. Nature Commun. 5, 4722 (2014)
Lam, A. J. et al. Improving FRET dynamic range with bright green and red fluorescent proteins. Nature Methods 9, 1005–1012 (2012)
Hajdukiewicz, P., Svab, Z. & Maliga, P. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 25, 989–994 (1994)
Curtis, M. D. & Grossniklaus, U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133, 462–469 (2003)
Susaki, D., Takeuchi, H., Tsutsui, H., Kurihara, D. & Higashiyama, T. Live imaging and laser disruption reveal the dynamics and cell-cell communication during Torenia fournieri female gametophyte development. Plant Cell Physiol. 56, 1031–1041 (2015)
Maruyama, D. et al. Independent control by each female gamete prevents the attraction of multiple pollen tubes. Dev. Cell 25, 317–323 (2013)
Nagahara, S., Takeuchi, H. & Higashiyama, T. Generation of a homozygous fertilization-defective gcs1 mutant by heat-inducible removal of a rescue gene. Plant Reprod. 28, 33–46 (2015)
Guex, N., Peitsch, M. C. & Schwede, T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: a historical perspective. Electrophoresis 30, S162–S173 (2009)
Miyazaki, S. et al. ANXUR1 and 2, sister genes to FERONIA/SIRENE, are male factors for coordinated fertilization. Curr. Biol. 19, 1327–1331 (2009)
Wrzaczek, M. et al. GRIM REAPER peptide binds to receptor kinase PRK5 to trigger cell death in Arabidopsis. EMBO J. 34, 55–66 (2015)
Acknowledgements
We thank M. Hasebe and S. Miyazaki for seeds of a part of mutant lines; Y. Matsubayashi, H. Shinohara, D. Maruyama, M. Ohtsu and K. Motomura for valuable comments and discussions; D. Kurihara, Y. Hamamura and S. Nagahara for technical assistance with confocal microscopy and physiological analyses; M. M. Kanaoka for agro-infiltration method using tobacco leaf; S. Oishi for reverse-phase high-pressure liquid chromatography (HPLC); and the Japan Advanced Plant Science Network for use of some microscopes. This work was supported by grants from the Japan Science and Technology Agency (ERATO project to T.H.) and the Japan Society for the Promotion of Science Fellowships (no. 5834 to H.T.).
Author information
Authors and Affiliations
Contributions
H.T. designed the study; H.T. performed experiments; H.T. and T.H. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
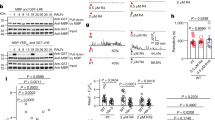
Extended Data Figure 1 Phylogenetic relationship, expression, gene structure and fertility of A. thaliana PRK family protein mutants.
a, A neighbour-joining (NJ) tree constructed using full-length amino-acid sequences of PRK1–PRK6 (ref. 14), PRK7 and PRK8 (assigned in this study). The bootstrap values as percentages and the scale for substitutions per site are shown. b, PRK expression during pollen germination and growth. Expression levels are shown using normalized values and standard deviation from microarray data (n = 4 for dry pollen, 30 min in vitro pollen tube (PT), and 4 h in vitro PT; n = 3 for semi-in-vivo PT)13. c, Structure and T-DNA insertion of PRK genes. Grey boxes show exons, and black boxes show introns or untranslated regions that are registered in The Arabidopsis Information Resource (TAIR). The T-DNA insertion sites determined by genomic PCR and sequencing are drawn on the gene structure and indicated in Extended Data Table 1. d, Reverse transcription PCR (RT–PCR) analysis of the prk single mutants. Anther cDNA was used for the analysis. Positions of the primers are indicated in the gene structure (c). ACT2 was used as the loading control. For gel source data, see Supplementary Fig. 1. e, f, The rate of developing seeds upon self-pollination of prk single mutants (e) and upon reciprocal crosses with Col-0 and prk multiple mutants (f). Asterisks in e indicate the mutants used for the prk multiple mutants in this study. Note that, in addition to multiple mutants of PRK1 and PRK3 subclass genes (shown in dark blue), multiple mutants of PRK1, PRK4 and PRK6, which are the top three most highly expressed in semi-in-vivo pollen tubes, and PRK1, PRK2, PRK4 and PRK5, which form another subclade, were analysed. The prk1 prk2 prk4 prk5 multiple mutant contains prk1 prk2 prk5 mutations that cause reduced pollen tube growth in vitro14. Data are mean and s.d. of three (all samples in e; Col-0 pistil × prk3-1 prk6-1 pr8-1 and prk3-1 prk6-1 pr8-2 in f) or four (other samples in f) pistils. g, Developing seeds in siliques 8 days after pollination with Col-0, prk6 and the prk1 prk3 prk6 triple mutant. The images are representative of four samples. Scale bar, 1 mm.
Extended Data Figure 2 Evaluation of AtLURE1-responsive wavy assay.
a–f, Semi-in-vivo pollen tubes on medium containing the indicated concentrations of AtLURE1.2 peptide. Entire (a–d) and magnified (e, f) images show wavy and swollen tip growth of wild-type, but not prk6-1 mutant, pollen tubes in a concentration-dependent manner. Scale bars, 200 μm (a–d) and 20 μm (e, f). g, Growth of PRK6–mRuby2 pollen tubes that directly germinated on medium (that is, in vitro pollen tubes) containing 1 μM AtLURE1.2 peptide. h, Growth of semi-in-vivo pollen tubes from chx21-s1/chx21-s1 chx23-4/CHX23 plant18 on medium containing 1 μM AtLURE1.2 peptide. Roughly half the pollen tubes showed wavy growth as in the wild type (arrowheads), but the rest did not (arrows). These results indicate that in vitro pollen tubes and chx21 chx23 double-mutant pollen tubes have no or less ability to respond to the external AtLURE1 peptide. Scale bars, 200 μm. i, Semi-in-vivo pollen tube growth and AtLURE1-responsive wavy assay for prk mutants additional to those shown in Fig. 1f. Scale bars, 100 μm (top) and 10 μm (bottom). j, Complementation of the growth defect in prk3 prk6 pollen tubes by expression of PRK3–mClover or PRK6–mClover. Note that PRK3–mClover expression restored the growth defect but not the wavy response. The images of a–j are representative of at least three assays. k, Pollen tube tip localization of PRK3–mClover in a single-plane confocal image (top) and its intensity image by pseudocolour (bottom). The data are representative of three samples. Scale bar, 10 μm.
Extended Data Figure 3 Pollen tube growth of prk mutants in the pistil.
a, Pollen tubes of Col-0, prk6, prk3 prk6 and prk1 prk3 prk6 growing in the Col-0 pistils. Aniline blue staining was performed 12 or 24 HAP. White arrows indicate the tip of the longest pollen tube in the transmitting tract. Data are representative of three samples for each genotype. Scale bar, 500 μm. b, Length from the top of the stigma to the tip of the longest pollen tube, 12 or 24 HAP with Col-0 and prk mutants. About 2,700 μm is the maximum limit for the length in this measurement. The data are the mean and s.d. of three pistils. ND, no data.
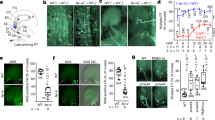
Extended Data Figure 4 Growth and ovule-targeting of prk mutant pollen tubes on the septum surface.
a–g, Entire images of growth and ovule-targeting of wild-type (a), prk6 (b), prk3 prk6 (c, d), prk3 prk6 prk8-2 (e, f), and prk1 prk3 prk6 (g) pollen tubes on the septum surface in wild-type pistils. Arrows indicate the tip of the longest pollen tube on the septum surface. Asterisks mark ovules that did not attract near pollen tubes. The regions shown in a, b and d are shown in Fig. 2e, f and g, respectively, as higher magnification images. Data are representative of 1–3 images for each genotype. Similar growth properties were observed in a total of 4 samples. Scale bar, 500 μm. Quantitative analysis is shown in Fig. 2d. No analysis was performed for the prk1 prk3 prk6 mutant because almost no pollen tube reached the ovule.
Extended Data Figure 5 Interaction of PRK6 with pollen-expressed ROPGEFs, PRKs and LIPs.
a, Gene expression of ROPGEFs during pollen germination and growth. The data are normalized expression values and standard deviation from microarray data (n = 4 for dry pollen, 30 min in vitro PT, and 4 h in vitro PT; n = 3 for semi-in-vivo PT)13 as noted in Extended Data Fig. 1b. ROPGEF8, ROPGEF9, ROPGEF11, ROPGEF12 and ROPGEF13 are expressed specifically in the dry pollen grain and pollen tube. b, BiFC assay showing the interaction between PRK6–cYFP and nYFP–GEF8, nYFP–GEF9, nYFP–GEF12, or nYFP–GEF13 (see Methods). c, A control experiment using C-terminal-deleted ROPGEF12 (ROPGEF12ΔC). The C-terminal domain is suggested to mediate the interaction with PRK2 (ref. 8). d, BiFC assay showing interaction between PRK6–cYFP and PRK6–nYFP, PRK3–nYFP, LIP1–nYFP or LIP2–nYFP. Scale bars, 50 μm. Images are representative of more than three experiments. e, Co-immunoprecipitation assay of PRK–mClover and ROPGEF12 proteins expressed in N. benthamiana leaf cells. ROPGEF12-3 × Flag protein was precipitated with full-length PRK3, PRK6 and kinase domain-deleted PRK6 (K-del), but not mClover control or cytosolic domain-deleted PRK6 (cyto-del-1). Data are representative of three experiments. For gel source data, see Supplementary Fig. 2.
Extended Data Figure 6 PRK6 protein structure and PRK proteins of A. thaliana, A. lyrata and C. rubella.
a, Structures of the PRK6 protein and its deletion version used in this study. The PRK6 extracellular domain contains the N-terminal cap and six LRRs. JM, juxtamembrane domain; N-cap, N-terminal cap; SP, signal peptide; TM, transmembrane domain. The numbers indicate the amino acid ranges of each domain. b, A 3D ribbon model of the PRK6 extracellular domain, amino acid residues 28–231, was predicted using the homology modelling platform, SWISS-MODEL (http://swissmodel.expasy.org/), and the FLS2 crystal structure (Protein Data Bank (PDB) accession 4MN8) as a template, and was drawn using Swiss-PdbViewer (http://spdbv.vital-it.ch/)38. The PRK6 extracellular domain contains the N-terminal cap and six LRRs. c, A neighbour-joining tree constructed using PRK protein sequences from tomato (Lycopersicon esculentum, LePRK1-3), A. thaliana (AtPRK1–AtPRK8), A. lyrata (AlPRK1–AlPRK8), and C. rubella (CrPRK1–CrPRK8). The bootstrap values as percentages and the scale for substitutions per site are shown. Accession numbers for A. lyrata and C. rubella PRKs: AlPRK1 (XP_002868416), AlPRK2 (XP_002883746), AlPRK3 (XP_002877261), AlPRK4 (XP_002883234), AlPRK5 (XP_002891583), AlPRK6 (XP_002871954), AlPRK7 (XP_002867307, modified according to the genome sequence), AlPRK8 (XP_002887434), CrPRK1 (EOA19015), CrPRK2 (EOA32286, partial sequence), CrPRK3 (EOA25493), CrPRK4 (EOA31871), CrPRK5 (EOA37472), CrPRK6 (EOA23063), CrPRK7 (EOA18255), and CrPRK8 (EOA34527). d, A sequence alignment of AtPRK3, AtPRK6 and CrPRK6. Signal peptide, N-terminal cap, LRR1–LRR6, transmembrane domain and kinase domain are indicated beneath the alignment.
Extended Data Figure 7 Semi-in-vivo pollen tube growth and response to the AtLURE1 peptide of PRK6 variant mutants.
Pollen tubes of prk6, prk3 prk6, prk3 prk6 prk8-2 and prk1 prk3 prk6 mutants were assessed in this assay. Full-length PRK6, the PRK6 orthologue of C. rubella (CrPRK6), kinase-domain-deleted PRK6 (K-del), and cytosolic-domain-deleted PRK6 (cyto-del-2) were expressed as mRuby2 fusion proteins under the control of their own promoters. Upper differential interference contrast images show semi-in-vivo pollen tube growth in the medium containing the AtLURE1 peptide at 6 HAP. Yellow arrowheads mark some of the pollen tubes showing apparent wavy phenotype. The bottom two images are a blight field image and a confocal image for mRuby2 of a representative pollen tube in the wavy assay. The data are representative images of at least three assays for one or two lines of each genotype. Scale bars, 200 μm (top) and 20 μm (bottom).
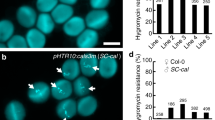
Extended Data Figure 8 A conserved basic amino acid patch of LURE is essential for attraction.
a, The sequence of full-length AtLURE1.2 accompanied by lysine/arginine residues (yellow highlight) mutated to glycines for AtLURE1.2(GGGG). Cysteine residues in the mature peptide are shown in red. b, c, Semi-in-vivo attraction assay using gelatine beads containing 5 μM His–AtLURE1.2(GGGG) (b) and wavy assay using 10 μM His–AtLURE1.2(GGGG) in the medium (c). The AtLURE1.2(GGGG) peptide showed no activity in these assays. The data are representative of 14 or 3 samples for b or c, respectively. Scale bars, 20 μm (b) and 200 μm (c).
Supplementary information
Supplementary Information
This file contains a Supplementary Discussion and Supplementary Figures 1-2 which show uncropped scans with size marker indications. (PDF 289 kb)
Supplementary Table 1
This table contains a list of primers used in the study. (XLS 34 kb)
Time lapse imaging of PRK6 mRuby2 during pollen tube tip growth
PRK6 mRuby2 was observed predominantly at the plasma membrane of the tip and detected in cytoplasmic granules with cytoplasmic streaming. Scale bar, 10 μm. (MOV 1215 kb)
Time lapse imaging of semi-in vivo pollen tube growth and response to the AtLURE1 peptide
Pollen tubes of wild-type (Col-0), prk6, and prk6 expressing PRK6-mRuby2 (PRK6-mRuby2) grew straight in the absence of AtLURE1 peptides in the medium (-). In this observation, the pollen tube growth rate at 3-4 h after pollination (180-240 min) was 200 ± 25 µm/h in the wild type and 151 ± 18 µm/h in prk6. Col-0 and PRK6-mRuby2, but not prk6, showed the wavy growth phenotype in the presence of 1 µM AtLURE1.2 in the medium (+). (MOV 2319 kb)
Time lapse imaging of PRK6 mRuby2 and Alexa488-labeled AtLURE1.2 during pollen tube reorientation
Time-lapse images captured every 5 s shown in Fig. 4a-f. Overlaid images of PRK6-mRuby2 and Alexa488-labeled AtLURE1.2 (left) and intensity images for PRK6-mRuby2 (right) are shown. A gelatine bead containing 5 µM Alexa488-labeled AtLURE1.2 was used in this assay. Scale bar, 10 μm. (MOV 3680 kb)
An additional example of time lapse imaging of PRK6 mRuby2 and Alexa488-labeled AtLURE1.2 during pollen tube reorientation
An additional example of the assay shown in Supplementary Video 3. Scale bar, 10 μm. (MOV 5087 kb)
Rights and permissions
About this article
Cite this article
Takeuchi, H., Higashiyama, T. Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature 531, 245–248 (2016). https://doi.org/10.1038/nature17413
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature17413
This article is cited by
-
Synergid cell calcium oscillations refine understanding of FERONIA/LORELEI signaling during interspecific hybridization
Plant Reproduction (2024)
-
Pollen tube emergence is mediated by ovary-expressed ALCATRAZ in cucumber
Nature Communications (2023)
-
Two aspartic proteases, BnaAP36s and BnaAP39s, regulate pollen tube guidance in Brassica napus
Molecular Breeding (2023)
-
Whole-mount RNA in situ hybridization technique in Torenia ovules
Plant Reproduction (2023)
-
Insights into pollen–stigma recognition: self-incompatibility mechanisms serve as interspecies barriers in Brassicaceae?
aBIOTECH (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.