Abstract
The eukaryotic exosome is a conserved RNA-degrading complex that functions in RNA surveillance, turnover and processing. How the same machinery can either completely degrade or precisely trim RNA substrates has long remained unexplained. Here we report the crystal structures of a yeast nuclear exosome containing the 9-subunit core, the 3′–5′ RNases Rrp44 and Rrp6, and the obligate Rrp6-binding partner Rrp47 in complex with different RNAs. The combined structural and biochemical data of this 12-subunit complex reveal how a single-stranded RNA can reach the Rrp44 or Rrp6 active sites directly or can bind Rrp6 and be threaded via the central channel towards the distal RNase Rrp44. When a bulky RNA is stalled at the entrance of the channel, Rrp6–Rrp47 swings open. The results suggest how the same molecular machine can coordinate processive degradation and partial trimming in an RNA-dependent manner by a concerted swinging mechanism of the two RNase subunits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mitchell, P., Petfalski, E., Shevchenko, A., Mann, M. & Tollervey, D. The exosome: a conserved eukaryotic RNA processing complex containing multiple 3′→5′ exoribonucleases. Cell 91, 457–466 (1997)
Januszyk, K. & Lima, C. D. The eukaryotic RNA exosome. Curr. Opin. Struct. Biol. 24, 132–140 (2014)
Chlebowski, A., Lubas, M., Jensen, T. H. & Dziembowski, A. RNA decay machines: the exosome. Biochim. Biophys. Acta 1829, 552–560 (2013)
Makino, D. L., Halbach, F. & Conti, E. The RNA exosome and proteasome: common principles of degradation control. Nature Rev. Mol. Cell Biol. 14, 654–660 (2013)
Houseley, J. & Tollervey, D. The many pathways of RNA degradation. Cell 136, 763–776 (2009)
Allmang, C. et al. Functions of the exosome in rRNA, snoRNA and snRNA synthesis. EMBO J. 18, 5399–5410 (1999)
Wyers, F. et al. Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell 121, 725–737 (2005)
Preker, P. et al. RNA exosome depletion reveals transcription upstream of active human promoters. Science 322, 1851–1854 (2008)
Porrua, O. & Libri, D. RNA quality control in the nucleus: the Angels’ share of RNA. Biochim. Biophys. Acta 1829, 604–611 (2013)
Schaeffer, D., Clark, A., Klauer, A. A., Tsanova, B. & van Hoof, A. Functions of the cytoplasmic exosome. Adv. Exp. Med. Biol. 702, 79–90 (2011)
Dziembowski, A., Lorentzen, E., Conti, E. & Seraphin, B. A single subunit, Dis3, is essentially responsible for yeast exosome core activity. Nature Struct. Mol. Biol. 14, 15–22 (2007)
Liu, Q., Greimann, J. C. & Lima, C. D. Reconstitution, activities, and structure of the eukaryotic RNA exosome. Cell 127, 1223–1237 (2006)
Makino, D. L., Baumgärtner, M. & Conti, E. Crystal structure of an RNA-bound 11-subunit eukaryotic exosome complex. Nature 495, 70–75 (2013)
Bonneau, F., Basquin, J., Ebert, J., Lorentzen, E. & Conti, E. The yeast exosome functions as a macromolecular cage to channel RNA substrates for degradation. Cell 139, 547–559 (2009)
Drazkowska, K. et al. The RNA exosome complex central channel controls both exonuclease and endonuclease Dis3 activities in vivo and in vitro. Nucleic Acids Res. 41, 3845–3858 (2013)
Schneider, C., Kudla, G., Wlotzka, W., Tuck, A. & Tollervey, D. Transcriptome-wide analysis of exosome targets. Mol. Cell 48, 422–433 (2012)
Wasmuth, E. V. & Lima, C. D. Exo- and endoribonucleolytic activities of yeast cytoplasmic and nuclear RNA exosomes are dependent on the noncatalytic core and central channel. Mol. Cell 48, 133–144 (2012)
Liu, J.-J. et al. Visualization of distinct substrate-recruitment pathways in the yeast exosome by EM. Nature Struct. Mol. Biol. 21, 95–102 (2014)
Butler, J. S. & Mitchell, P. Rrp6, Rrp47 and cofactors of the nuclear exosome. Adv. Exp. Med. Biol. 702, 91–104 (2011)
Schuch, B. et al. The exosome-binding factors Rrp6 and Rrp47 form a composite surface for recruiting the Mtr4 helicase. EMBO J. 33, 2829–2846 (2014)
Briggs, M. W., Burkard, K. T. & Butler, J. S. Rrp6p, the yeast homologue of the human PM-Scl 100-kDa autoantigen, is essential for efficient 5.8 S rRNA 3′ end formation. J. Biol. Chem. 273, 13255–13263 (1998)
Wasmuth, E. V., Januszyk, K. & Lima, C. D. Structure of an Rrp6-RNA exosome complex bound to poly(A) RNA. Nature 511, 435–439 (2014)
Lorentzen, E., Basquin, J., Tomecki, R., Dziembowski, A. & Conti, E. Structure of the active subunit of the yeast exosome core, Rrp44: diverse modes of substrate recruitment in the RNase II nuclease family. Mol. Cell 29, 717–728 (2008)
Midtgaard, S. F. et al. Structure of the nuclear exosome component Rrp6p reveals an interplay between the active site and the HRDC domain. Proc. Natl Acad. Sci. USA 103, 11898–11903 (2006)
Callahan, K. P. & Butler, J. S. Evidence for core exosome independent function of the nuclear exoribonuclease Rrp6p. Nucleic Acids Res. 36, 6645–6655 (2008)
Arraiano, C. M., Mauxion, F., Viegas, S. C., Matos, R. G. & Seraphin, B. Intracellular ribonucleases involved in transcript processing and decay: precision tools for RNA. Biochim. Biophys. Acta 1829, 491–513 (2013)
Malet, H. et al. RNA channelling by the eukaryotic exosome. EMBO Rep. 11, 936–942 (2010)
Januszyk, K., Liu, Q. & Lima, C. D. Activities of human RRP6 and structure of the human RRP6 catalytic domain. RNA 17, 1566–1577 (2011)
Ben-Shem, A. et al. The structure of the eukaryotic ribosome at 3.0 Å resolution. Science 334, 1524–1529 (2011)
Klinge, S., Voigts-Hoffmann, F., Leibundgut, M., Arpagaus, S. & Ban, N. Crystal structure of the eukaryotic 60S ribosomal subunit in complex with initiation factor 6. Science 334, 941–948 (2011)
Kabsch, W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. D 66, 133–144 (2010)
Vonrhein, C. et al. Data processing and analysis with the autoPROC toolbox. Acta Crystallogr. D 67, 293–302 (2011)
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D 67, 235–242 (2011)
Evans, P. R. & Murshudov, G. N. How good are my data and what is the resolution? Acta Crystallogr. D 69, 1204–1214 (2013)
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010)
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007)
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010)
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010)
Terwilliger, T. C. et al. Decision-making in structure solution using Bayesian estimates of map quality: the PHENIX AutoSol wizard. Acta Crystallogr. D 65, 582–601 (2009)
Cowtan, K. Fitting molecular fragments into electron density. Acta Crystallogr. D 64, 83–89 (2008)
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D 68, 352–367 (2012)
Vincent, H. A. & Deutscher, M. P. Substrate recognition and catalysis by the exoribonuclease RNase R. J. Biol. Chem. 281, 29769–29775 (2006)
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9, 671–675 (2012)
Acknowledgements
We would like to thank the MPIB Crystallization Facility and MPIB Core Facility; the beamline scientists at SLS beamlines PXII and PXIII and APS beamline 17-ID-B for assistance with data collection; M. Krause for the schematic drawing; F. Bonneau and J. Gagneur (Gene Center) for help with statistical analyses, members of our lab for comments and discussion. This study was supported by the Max Planck Gesellschaft, the European Commission (ERC Advanced Investigator Grant 294371 and Marie Curie ITN RNPnet), the Deutsche Forschungsgemeinschaft (DFG SFB646, SFB1035, GRK1721, FOR1680 and CIPSM) and the Louis Jeantet Foundation to E.C.
Author information
Authors and Affiliations
Contributions
D.L.M, B.S. and E.C. designed the experiments, D.L.M solved the structures of Exo-12–(A/U)18 and Exo-12–duplexU31, B.S. solved the structure of Rrp6iexo–U15 and performed the nuclease assays, B.S. and C.B did the FRET experiments, E.S. and M.B. assisted with purification of exosome subunits, E.C., D.L.M. and B.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
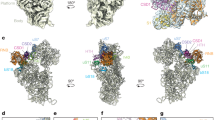
Extended Data Figure 1 Comparison of Rrp44 and Rrp6 conformations in exosome structures.
a, Schematic diagrams of the multi-domain organization of Rrp44 (in pink) and Rrp6 (in red). The individual domains and their functions discussed in the text are labelled. The exoribonuclease regions (exo) are indicated. In white is the low complexity nuclear localization signal (NLS)-containing region of Rrp6 that is not present in the current crystal structures. b, Conformations of Rrp44 domains in Exo-12 (pink, direct RNA route) and in a previous structure (grey, channel-dependent RNA route)13 after superposition of Exo-9–Rrp44PIN. To appreciate the conformational change, the same helix in Rrp44exo in the two structures is highlighted (dark and light cyan) (see also panel c). The position of the PIN domain active site is indicated (with the mutated residue D171N in stick representation). c, Structures of Exo-9–Rrp44–Rrp6–Rrp47–(A/U)18 (this work), Exo-9–Rrp44–Rrp6–Rrp47–duplexU31 (this work), Exo-9–Rrp6C-exo–A24 (PDB 4OO1) and Exo-9–Rrp44–Rrp6C–duplexU31 (PDB 4IFD) are shown in the same two orientations, related by a 90° rotation around a vertical axis.
Extended Data Figure 2 Structural and biochemical properties of the Rrp6 exoribonuclease.
a, The exoribonuclease region of Rrp6 is a rather rigid unit. Superposition of the Rrp6exo subunits of Rrp6exo–U15 (in red, this work), of Exo-9–Rrp44–Rrp6–Rrp47 (Exo-12)–(A/U)18 (dark grey, this work), and of Exo-9–Rrp6C-exo–A24 (light grey, PDB 4OO1). The HRDC and DEDD-Y domains of Rrp6exo and the intricate loops in between have similar conformations. b, RNA path from Rrp6iexo in RNA-binding mode to the central channel. Magnified views of the superposition of Rrp6iexo–U15 in RNA-binding mode (coloured as in Fig. 2a) on Rrp6i in the Exo-12–(A/U)18 structure (proteins coloured as in Fig. 1, RNA in grey, and Exo-10 in a semi-transparent surface representation). Views are related by a 45° rotation around the vertical axis. c, RNA path of Rrp6iexo in RNA-degrading mode versus Exo-12–(A/U)18 RNA (RNA-binding mode). Magnified views of the superposition of Rrp6iexo–U15 in RNA-degrading mode (coloured as in Fig. 3a) on Rrp6i in the Exo-12–(A/U)18 structure (proteins coloured as in Fig. 1, RNA in grey, and Exo-10 in a semi-transparent surface representation). d, RNA-degradation activity of catalytically active Rrp6–Rrp47N mutants alone and in context of Exo-12 towards GC-rich duplex RNA with a 3′ A35 overhang (ds17A35; left panel) and a 3′ A10 overhang (ds17A10, a full-view copy of Fig. 3c; middle panel). A Coomassie-stained SDS–PAGE gel of the respective proteins is shown on the right. The 35 nucleotide single-stranded 3′ overhang of ds17A35 is able to reach Rrp44exo via the exosome channel whereas the 3′ overhang of ds17A10 is too short to reach Rrp44exo. Shown are representative gels of three technical replicates (n = 3).
Extended Data Figure 3 Influence of Rrp6 mutants on Rrrp44 exoribonuclease activity.
a, Left panel, RNA degradation assay of Exo-10 in presence of catalytically inactive Rrp6i–Rrp47N mutants towards single-stranded RNA (ss17A35). Right panels: top, Coomassie-stained gel with input protein samples; bottom, quantification of the fraction of remaining substrate (average of three technical replicates (n = 3), error bars represent s.d.). b–e, Comparison of the RNA decay rates. The RNA decay rate of Exo-9–Rrp44 in the presence of Rrp6i–Rrp47N (blue) is significantly different (P < 1 × 10−5, F-test) from decay rates in the presence of Rrp6i(Y244A;R245A)–Rrp47N (purple, b), Rrp6i(Y244A;R245A;F294A;Y315A)–Rrp47N (green, c) and in the absence of Rrp6i–Rrp47N (red, d). The decay rate in presence of Rrp6i(F294A;Y315A)–Rrp47N (orange, e) shows no significant difference. Individual points for each of the three experiments are coloured and represented with the same symbols as above in a.
Extended Data Figure 4 Interplay of Rrp6 and Rrp44 in RNA degradation.
Schematic drawing of the sequence of events leading to RNA degradation by the 12-subunit nuclear exosome (drawn in a longitudinal section, showing the inner central channel). RNA binds at the top of Rrp6–Rrp47 and stochastically reaches the distributive active site of Rrp6 (left panel). At steady state, the RNA is channelled to the processive active site of Rrp44 (right panel) and Rrp6 functions only in RNA-binding mode. The expectation is that in the absence of RNA (left panel), Rrp44 can be in an open conformation, which allows direct access of 3′ single-stranded RNA.
Extended Data Figure 5 Rrp6–Rrp47 is in an open conformation in the Exo-12–duplexU31 structure.
a, Snapshot of the electron density (2mFo − DFc, contoured at 0.8σ) around the DEDD-Y domain of Rrp6 in the Exo-12–duplexU31 structure. b, Silver-stained SDS–PAGE gel showing the content of the Exo-12–duplexU31 crystals. The crystals were extensively washed (three successive washes, lanes W1, W2 and W3) before dissolving them and running them on the gel (Xtal lane). The protein sample used in crystallization experiments is shown on the left (Prot). c, Superposition of both Exo-12 structures shows that the presence of the 5′ duplex RNA (in semi-transparent surface representation) in the Exo-12–duplexU31 structure would sterically clash at the loop regions that connect the HRDC and DEDD-Y domain of Rrp6 in the Exo-12–(A/U)18 structure.
Extended Data Figure 6 FRET-based detection of Rrp6 conformational changes in Exo-9–Rrp44–Rrp6 (Exo-11).
a, Coomassie-stained gel of the reconstituted eCFP/eYFP-labelled exosome complexes. b, FRET efficiency (dark red) of Exo-11–eCFP–eYFP (Exo-10–eYFP–eCFP–Rrp6iexo-C) fusion complex upon excitation at 429 nm. Control emission spectra for Exo-11–eCFP (cyan) and Exo-11–eYFP (yellow). c, FRET efficiency of Exo-11–eCFP–eYFP in the presence of a 2.5-fold molar excess of (A/U)18 RNA (orange). d, FRET efficiency of Exo-11–eCFP–eYFP in the presence of a 2.5-fold molar excess of duplexU31 RNA (blue).
Extended Data Figure 7 Handover from Rrp44-mediated degradation to Rrp6-mediated trimming.
Model of the sequence of events leading to exosome-mediated 5.8S rRNA processing. Within the pre-60S ribosomal particle, the 5.8S-152 rRNA precursor (generated from endonucleolytic cleavage of 7S rRNA) is targeted to the exosome (drawn in a longitudinal section, with the inner central channel highlighted). The 152 nucleotide extension6 is first stochastically degraded by the distributive activity of Rrp6 (Extended Data Fig. 4) and then threaded through the central channel to the processive exoribonuclease site of Rrp44 (left panel). At about 5.8S-30, the 3′-end extension is entirely embedded in the central channel, and the bulk of pre-60S would sterically clash with Rrp6–Rrp47. As a result, Rrp6–Rrp47, and probably also Rrp44, undergo conformational changes and contribute, together with Mtr4 and Mpp6, to extract the 30-nucleotide extension, which is then trimmed by Rrp6. This step would require the pre-ribosome to be kept in the vicinity of Rrp6–Rrp47–Mtr4–Mpp6 by additional interactions. Such a mechanism involves an open conformation of Rrp6–Rrp47 that is detached from the core. We note that the Rrp6ΔC mutant (lacking the C-terminal Exo-9 binding region) is still capable of performing the Rrp6-mediated step in 5.8S rRNA biogenesis25. In this mutant, Rrp6 is expected to detach from Exo-9–Rrp44 and to form a ternary Rrp6–Rrp47–Mtr4 complex. It is possible that such a sub-complex is still able to target the pre-ribosome, allowing the 152-nucleotide extension to be degraded by Rrp6.
Rights and permissions
About this article
Cite this article
Makino, D., Schuch, B., Stegmann, E. et al. RNA degradation paths in a 12-subunit nuclear exosome complex. Nature 524, 54–58 (2015). https://doi.org/10.1038/nature14865
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14865
This article is cited by
-
Hypoxia-driven deSUMOylation of EXOSC10 promotes adaptive changes in the transcriptome profile
Cellular and Molecular Life Sciences (2024)
-
EXOSC9 depletion attenuates P-body formation, stress resistance, and tumorigenicity of cancer cells
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.