Abstract
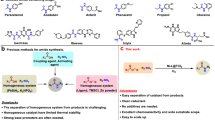
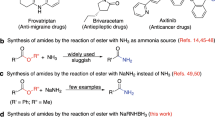
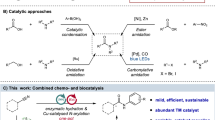
Amides are common functional groups that have been studied for more than a century1. They are the key building blocks of proteins and are present in a broad range of other natural and synthetic compounds. Amides are known to be poor electrophiles, which is typically attributed to the resonance stability of the amide bond1,2. Although amides can readily be cleaved by enzymes such as proteases3, it is difficult to selectively break the carbon–nitrogen bond of an amide using synthetic chemistry. Here we demonstrate that amide carbon–nitrogen bonds can be activated and cleaved using nickel catalysts. We use this methodology to convert amides to esters, which is a challenging and underdeveloped transformation. The reaction methodology proceeds under exceptionally mild reaction conditions, and avoids the use of a large excess of an alcohol nucleophile. Density functional theory calculations provide insight into the thermodynamics and catalytic cycle of the amide-to-ester transformation. Our results provide a way to harness amide functional groups as synthetic building blocks and are expected to lead to the further use of amides in the construction of carbon–heteroatom or carbon–carbon bonds using non-precious-metal catalysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Greenberg, A., Breneman, C. M. & Liebman, J. F. (eds) The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science (Wiley, 2003)
Pauling, L., Corey, R. B. & Branson, H. R. The structure of proteins: two hydrogen-bonded helical configurations of the polypeptide chain. Proc. Natl Acad. Sci. USA 37, 205–211 (1951)
Brix, K. & Stöcker, W. (eds) Proteases: Structure and Function (Springer, 2013)
Corey, E. J. & Cheng, X.-M. The Logic of Chemical Synthesis (Wiley, 1995)
Hudlicky, T. & Reed, J. W. The Way of Synthesis: Evolution of Design and Methods for Natural Products (Wiley, 2007)
Van Vranken, D. L. &. Weiss. G. A. Introduction to Bioorganic Chemistry and Chemical Biology (Garland Science, 2013)
Spletstoser, J. T., White, J. M., Tunoori, A. R. & Georg, G. I. Mild and selective hydrozirconation of amides to aldehydes using Cp2Zr(H)Cl: scope and mechanistic insight. J. Am. Chem. Soc. 129, 3408–3419 (2007)
Nahm, S. & Weinreb, S. M. N-methoxy-N-methylamides as effective acylating agents. Tetrahedron Lett. 22, 3815–3818 (1981)
Blangetti, M., Rosso, H., Prandi, C., Deagostino, A. & Venturello, P. Suzuki–Miyaura cross-coupling in acylation reactions, scope and recent developments. Molecules 18, 1188–1213 (2013)
Tatamidani, H., Kakiuchi, F. & Chatani, N. A new ketone synthesis by palladium-catalyzed cross-coupling reactions of esters with organoboron compounds. Org. Lett. 6, 3597–3599 (2004)
Dineen, T. A., Zajac, M. A. & Myers, A. G. Efficient transamidation of primary carboxamides by in situ activation with N,N-dialkylformamide dimethyl acetals. J. Am. Chem. Soc. 128, 16406–16409 (2006)
Stephenson, N. A., Zhu, J., Gellman, S. H. & Stahl, S. S. Catalytic transamidation reactions compatible with tertiary amide metathesis under ambient conditions. J. Am. Chem. Soc. 131, 10003–10008 (2009)
Keck, G. E., McLaws, M. D. & Wager, T. T. A direct and mild conversion of tertiary aryl amides to methyl esters using trimethyloxonium tetrafluoroborate: a very useful complement to directed metalation reactions. Tetrahedron 56, 9875–9883 (2000)
Nishimoto, S.-i., Izukawa, T. & Kagiya, T. Photo-induced ring-opening reactions of 1-(2-naphthoyl)aziridine in various solvents. Bull. Chem. Soc. Jpn 55, 1484–1488 (1982)
White, E. H. The chemistry of N-alkyl-N-nitrosoamides. II. A new method for the deamination of aliphatic amines. J. Am. Chem. Soc. 77, 6011–6014 (1955)
Guthrie, J. P., Pike, D. C. & Lee, Y.-C. Equilibrium constants and heats of formation of methyl esters and N,N-dimethyl amides of substituted benzoic acids. Can. J. Chem. 70, 1671–1683 (1992)
Tasker, S. Z., Standley, E. A. & Jamison, T. F. Recent advances in homogeneous nickel catalysis. Nature 509, 299–309 (2014)
Mesganaw, T. & Garg, N. K. Ni- and Fe-catalyzed cross-coupling reactions of phenol derivatives. Org. Process Res. Dev. 17, 29–39 (2013)
Rosen, B. M. et al. Nickel-catalyzed cross-couplings involving carbon–oxygen bonds. Chem. Rev. 111, 1346–1416 (2011)
Blakey, S. B. & MacMillan, D. W. C. The first Suzuki cross-couplings of aryltrimethylammonium salts. J. Am. Chem. Soc. 125, 6046–6047 (2003)
Zhang, X.-Q. & Wang, Z.-X. Nickel-catalyzed cross-coupling of aryltrimethylammonium triflates and amines. Org. Biomol. Chem. 12, 1448–1453 (2014)
Tobisu, M., Nakamura, K. & Chatani, N. Nickel-catalyzed reductive and borylative cleavage of aromatic carbon–nitrogen bonds in N-aryl amides and carbamates. J. Am. Chem. Soc. 136, 5587–5590 (2014)
Shiba, T., Kurahashi, T. & Matsubara, S. Nickel-catalyzed decarbonylative alkylidenation of phthalimides with trimethylsilyl-substituted alkynes. J. Am. Chem. Soc. 135, 13636–13639 (2013)
Quasdorf, K. W. et al. Suzuki–Miyaura cross-coupling of aryl carbamates and sulfamates: experimental and computational studies. J. Am. Chem. Soc. 133, 6352–6363 (2011)
Mesganaw, T. et al. Nickel-catalyzed amination of aryl carbamates and sequential site-selective cross-couplings. Chem. Sci. 2, 1766–1771 (2011)
Hong, X., Liang, Y. & Houk, K. N. Mechanisms and origins of switchable chemoselectivity of Ni-catalyzed C(aryl)–O and C(acyl)–O activation of aryl esters with phosphine ligands. J. Am. Chem. Soc. 136, 2017–2025 (2014)
Lu, Q., Yu, H. & Fu, Y. Mechanistic study of chemoselectivity in Ni-catalyzed coupling reactions between azoles and aryl carboxylates. J. Am. Chem. Soc. 136, 8252–8260 (2014)
Xu, H. et al. Key mechanistic features of Ni-catalyzed C–H/C–O biaryl coupling of azoles and naphthalene-2-yl pivalates. J. Am. Chem. Soc. 136, 14834–14844 (2014)
Yamamoto, T., Ishizu, J., Kohara, T., Komiya, S. & Yamamoto, A. Oxidative addition of aryl carboxylates to nickel(0) complexes involving cleavage of the acyl–oxygen bond. J. Am. Chem. Soc. 102, 3758–3764 (1980)
Amaike, K., Muto, K., Yamaguchi, J. & Itami, K. Decarbonylative C–H coupling of azoles and aryl esters: unprecedented nickel catalysis and application to the synthesis of muscoride A. J. Am. Chem. Soc. 134, 13573–13576 (2012)
Acknowledgements
We are grateful to Boehringer Ingelheim, DuPont, Bristol-Myers Squibb, the Camille and Henry Dreyfus Foundation, the A. P. Sloan Foundation, the S. T. Li Foundation, the University of California, Los Angeles (UCLA), and the NIH-NIGMS (grant number GM036700 to K.N.H.) for financial support. We are grateful to the NIH (grant number F31 GM101951-02 to N.F.F.N.), the NSF (grant number DGE-1144087 to E.L.B.), the Foote Family (L.H., T.K.S. and X.H.), and the ACS Division of Organic Chemistry (L.H.) for fellowship support. Computations were performed with resources made available by the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by the NSF (grant number OCI-1053575), as well as the UCLA Institute of Digital Research and Education (IDRE). This work was also supported by shared instrumentation grants from the NSF (grant number CHE-1048804) and the National Center for Research Resources (grant number S10RR025631).
Author information
Authors and Affiliations
Contributions
L.H., N.F.F.N., T.K.S., and E.L.B. designed and performed the experiments and analysed the experimental data; X.H., Y.-F.Y., and P.L. designed the computational studies and performed the analysis; K.N.H. and N.K.G. conceived and directed the investigations, and prepared the manuscript with contributions from all authors; all authors contributed to discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data – see Table of Contents for details. (PDF 15394 kb)
Rights and permissions
About this article
Cite this article
Hie, L., Fine Nathel, N., Shah, T. et al. Conversion of amides to esters by the nickel-catalysed activation of amide C–N bonds. Nature 524, 79–83 (2015). https://doi.org/10.1038/nature14615
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14615
This article is cited by
-
Amide C–N bonds activation by A new variant of bifunctional N-heterocyclic carbene
Nature Communications (2024)
-
Skeletal metalation of lactams through a carbonyl-to-nickel-exchange logic
Nature Communications (2023)
-
Deoxygenative alkylation of tertiary amides using alkyl iodides under visible light
Science China Chemistry (2022)
-
Binuclear Pd(II) complexes with multidentate Schiff base ligands: synthesis, catalysis, and antibacterial properties
Monatshefte für Chemie - Chemical Monthly (2022)
-
Direct alkylation of N,N-dialkyl benzamides with methyl sulfides under transition metal-free conditions
Communications Chemistry (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.