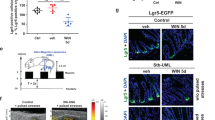
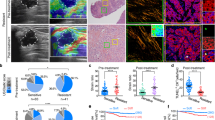
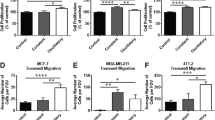
Abstract
The tumour microenvironment may contribute to tumorigenesis owing to mechanical forces such as fibrotic stiffness or mechanical pressure caused by the expansion of hyper-proliferative cells1,2. Here we explore the contribution of the mechanical pressure exerted by tumour growth onto non-tumorous adjacent epithelium. In the early stage of mouse colon tumour development in the Notch+Apc+/1638N mouse model, we observed mechanistic pressure stress in the non-tumorous epithelial cells caused by hyper-proliferative adjacent crypts overexpressing active Notch, which is associated with increased Ret and β-catenin signalling. We thus developed a method that allows the delivery of a defined mechanical pressure in vivo, by subcutaneously inserting a magnet close to the mouse colon. The implanted magnet generated a magnetic force on ultra-magnetic liposomes, stabilized in the mesenchymal cells of the connective tissue surrounding colonic crypts after intravenous injection. The magnetically induced pressure quantitatively mimicked the endogenous early tumour growth stress in the order of 1,200 Pa, without affecting tissue stiffness, as monitored by ultrasound strain imaging and shear wave elastography. The exertion of pressure mimicking that of tumour growth led to rapid Ret activation and downstream phosphorylation of β-catenin on Tyr654, imparing its interaction with the E-cadherin in adherens junctions, and which was followed by β-catenin nuclear translocation after 15 days. As a consequence, increased expression of β-catenin-target genes was observed at 1 month, together with crypt enlargement accompanying the formation of early tumorous aberrant crypt foci. Mechanical activation of the tumorigenic β-catenin pathway suggests unexplored modes of tumour propagation based on mechanical signalling pathways in healthy epithelial cells surrounding the tumour, which may contribute to tumour heterogeneity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
01 July 2015
Minor changes were made to the Extended Data Fig. 2 legend.
References
Ghajar, C. M. & Bissell, M. J. Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: insights from imaging. Histochem. Cell Biol. 130, 1105–1118 (2008)
Butcher, D. T., Alliston, T. & Weaver, V. M. A tense situation: forcing tumour progression. Nat. Rev. Cancer 9, 108–122 (2009)
Sawada, Y. et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127, 1015–1026 (2006)
Wozniak, M. A. & Chen, C. S. Mechano-tranduction: a growing role for contractibility. Nature Rev. Mol. Cell Biol. 10, 34–43 (2009)
Grashoff, C. et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266 (2010)
Mammoto, A. et al. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 457, 1103–1108 (2009)
Fre, S. et al. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc. Natl Acad. Sci. USA 106, 6309–6314 (2009)
Delarue, M. et al. Mechanical control of cell flow in multicellular spheroids. Phys. Rev. Lett. 110, 138103 (2013)
Stylianopoulos, T. et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl Acad. Sci. USA 109, 15101–15108 (2012)
Whitehead, J. et al. Mechanical factors activate β-catenin-dependent oncogene expression in APC mouse colon. HFSP J. 2, 286–294 (2008)
Jho, E. H. et al. Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 22, 1172–1183 (2002)
Kahlert, U. D. et al. Activation of canonical WNT/β-catenin signaling enhances in vitro motility of glioblastoma cells by activation of ZEB1 and other activators of epithelial-to-mesenchymal transition. Cancer Lett. 325, 42–53 (2012)
van Veelen, W. et al. β-catenin tyrosine 654 phosphorylation increases Wnt signalling and intestinal tumorigenesis. Gut 60, 1204–1212 (2011)
Clevers, H. Wnt/β-catenin signaling in development and disease. Cell 127, 469–480 (2006)
Smits, R. et al. Loss of Apc and the entire chromosome 18 but absence of mutations at the Ras and Tp53 genes in intestinal tumors from Apc1638N, a mouse model for Apc-driven carcinogenesis. Carcinogenesis 18, 321–327 (1997)
Stambolic, V. & Woodgett, J. R. Mitogen inactivation of glycogen synthase kinase-3 β in intact cells via serine 9 phosphorylation. Biochem. J. 303, 701–704 (1994)
Cohen, P. & Frame, S. The renaissance of GSK3. Nature Rev. Mol. Cell Biol. 2, 769–776 (2001)
Fodde, R. et al. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc. Natl Acad. Sci. USA 91, 8969–8973 (1994)
Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 17, 1247–1248 (1981)
Szoka, F., Jr & Papahadjopoulos, D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc. Natl Acad. Sci. USA 75, 4194–4198 (1978)
Béalle, G. et al. Ultra magnetic liposomes for MR imaging, targeting, and hyperthermia. Langmuir 28, 11834–11842 (2012)
Martina, M. S. et al. Generation of superparamagnetic liposomes revealed as highly efficient MRI contrast agents for in vivo imaging. J. Am. Chem. Soc. 127, 10676–10685 (2005)
Martina, M. S. et al. Magnetic targeting of rhodamine-labeled superparamagnetic liposomes to solid tumors: in vivo tracking by fibered confocal fluorescence microscopy. Mol. Imaging 6, 140–146 (2007)
Plassat, V., Martina, M. S., Barratt, G., Ménager, C. & Lesieur, S. Sterically stabilized superparamagnetic liposomes for MR imaging and cancer therapy: pharmacokinetics and biodistribution. Int. J. Pharm. 344, 118–127 (2007)
Martina, M.-S., Wilhelm, C. & Lesieur, S. The effect of magnetic targeting on the uptake of magnetic-fluid-loaded liposomes by human prostatic adenocarcinoma cells. Biomaterials 29, 4137–4145 (2008)
Plassat, V. et al. Anti-estrogen-loaded superparamagnetic liposomes for intracellular magnetic targeting and treatment of breast cancer tumors. Adv. Funct. Mater. 21, 83–92 (2011)
Bain, J., McLauchlan, H., Elliott, M. & Cohen, P. The specificities of protein kinase inhibitors: an update. Biochem. J. 371, 199–204 (2003)
Blake, R. A. et al. SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol. Cell. Biol. 20, 9018–9027 (2000)
Kim, D. W. et al. An orally administered multitarget tyrosine kinase inhibitor, SU11248, is a novel potent inhibitor of thyroid oncogenic RET/papillary thyroid cancer kinases. J. Clin. Endocrinol. Metab. 91, 4070–4076 (2006)
Langmuir, P. B. & Yver, A. Vandetanib for the treatment of thyroid cancer. Clin. Pharmacol. Ther. 91, 71–80 (2012)
Mologni, L., Redaelli, S., Morandi, A., Plaza-Menacho, I. & Gambacorti-Passerini, C. Ponatinib is a potent inhibitor of wild-type and drug-resistant gatekeeper mutant RET kinase. Mol. Cell. Endocrinol. 377, 1–6 (2013)
De Falco, V. et al. Ponatinib (AP24534) is a novel potent inhibitor of oncogenic RET mutants associated with thyroid cancer. J. Clin. Endocrinol. Metab. 98, E811–E819 (2013)
Gennisson, J. L. & Cloutier, G. Sol-gel transition in agar-gelatin mixtures studied with transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 53, 716–723 (2006)
Chamming’s, F., Latorre-Ossa, H., Lefrère-Belda, M. A., Fitoussi, V., Quibel, T., Assayag, F., Marangoni, E., Autret, G., Balvay, L., Pidial, L., Gennisson, J. L., Tanter, M., Cuenod, C. A., Clément, O. & Fournier, L. Shear wave elastography of tumor growth in a human breast cancer model with pathological correlation. Eur. Radiol. 23, 2079–2086 (2013)
Bercoff, J., Tanter, M. & Fink, M. Supersonic shear imaging: A new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 51, 396–409 (2004)
Sarvazyan, A. P., Rudenko, O. V., Swanson, S. D., Fowlkes, J. B. & Emelianov, S. Y. Shear wave elasticity imaging: A new ultrasonic technology of medical diagnostics. Ultrasound Med. Biol. 24, 1419–1435 (1998)
Swanson, C. A. et al. A comparison of diets of blacks and whites in three areas of the United States. Nutr. Cancer 20, 153–165 (1993)
Goss, S. A., Johnston, R. L. & Dunn, F. Comprehensive compilation of empirical ultrasonic properties of mammalian tissues. J. Acoust. Soc. Am. 64, 423–457 (1978)
Greenleaf, J. F., Fatemi, M. & Insana, M. Selected methods for imaging elastic properties of biological tissues. Annu. Rev. Biomed. Eng. 5, 57–78 (2003)
Sarvazyan, A. et al. Biophysical bases of elasticity imaging. Acoustical Imaging 21, 223–240 (1995)
Gennisson, J. L. et al. Viscoelastic and anisotropic mechanical properties of in vivo muscle tissue assessed by supersonic shear imaging. Ultrasound Med. Biol. 36, 789–801 (2010)
Royer, D., Gennisson, J. L., Deffieux, T. & Tanter, M. On the elasticity of transverse isotropic soft tissues. J. Acoust. Soc. Am. 129, 2757–2760 (2011)
Gennisson, J. L., Grenier, N., Combe, C. & Tanter, M. Supersonic shear wave elastography of in vivo pig kidney: influence of blood pressure, urinary pressure and tissue anisotropy. Ultrasound Med. Biol. 38, 1559–1567 (2012)
Bercoff, J. et al. in 2008 IEEE Ultrasonics Symposium, Vols 1-4 and Appendix Ultrasonics Symposium. 321–324 (2008)
Madsen, E. L. et al. Anthropomorphic breast phantoms for testing elastography systems. Ultrasound Med. Biol. 32, 857–874 (2006)
Ophir, J., Cespedes, I., Ponnekanti, H., Yazdi, Y. & Li, X. Elastography — a quantitative method for imaging the elasticity of biological tissues. Ultrason. Imaging 13, 111–134 (1991)
Latorre-Ossa, H., Gennisson, J.-L., De Brosses, E. & Tanter, M. Quantitative imaging of nonlinear shear modulus by combining static elastography and shear wave elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 59, 833–839 (2012)
Righetti, R. et al. The feasibility of using poroelastographic techniques for distinguishing between normal and lymphedematous tissues in vivo. Phys. Med. Biol. 52, 6525–6541 (2007)
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative CT method. Nature Protocols 3, 1101–1108 (2008)
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acid Res. 38, e164 (2010)
Levy, M. et al. Long term in vivo biotransformation of iron oxide nanoparticles. Biomaterials 32, 3988 (2011)
Acknowledgements
This research was funded by the CNRS, INSERM, ARC (grant no.s 5030 and 29324), ANR (grant no.s 09PIRI0013-01 and 11 BSV5014-01), the Labex CelTisPhyBio (grant no. 11-LBX-0038), Fonds CSP, Fondation Pierre-Gille de Gennes, Fondation de France and the Inca (grant no. PLBIO13-172). High-throughput sequencing was supported by the grants ANR-10-EQPX-03 and ANR10-INBS-09-08 from the Agence Nationale de la Recherche (investissements d’avenir) and by the Canceropôle Ile-de-France. We thank the members of the Animal House Facility of Institut Curie, particularly Stéphanie Boissel, Virginie Dangles-Marie and Isabelle Grandjean, the IC Imaging facility members of PICT-IBiSA at BDD (UMR3215/U934) and Cell and Tissue Imaging Facility (UMR144 CNRS), Olivier Renaud’s team and François Waharte, Thibaut Brunet for discussions on GSK3 phosphorylation, Pierre Gestraud for NGS analysis help, Jean-Michel Frapart and Sonia Lajnev for help in the RPE experiments, and Didier Meseure (IC hospital) for anathomo-pathological analyses of the mechanically induced tumours.
Author information
Authors and Affiliations
Contributions
M.E.F.-S. and S.B. designed and carried out experiments, interpreted data and prepared the manuscript. H.M. and S.L. produced the magnetic liposomes, G.B., A.M. and C.M. produced the ultra-magnetic liposomes, and M.E.F.-S. and S.B. contributed to their synthesis. M.E.F.-S., S.B. and C.R. performed the surgery of mice. L.F., A.C., M.E.F.-S., S.B. and C.H. set up the technique of implant the magnet and injected the mice. S.B., H.M. and S.L. measured, and defined respectively, the magnetic liposomes dosage administered intravenously to mice. M.E.F.-S., S.B., H.L., J.G. and M.T. performed acoustic experiments and interpreted the results. J.W. and M.E.F.-S. found the mechanical activation of pY1062 Ret kinase ex vivo. S.F. and S.R. provided all transgenic mice, designed and interpreted associated experiments. L.B. realized the colonoscopy with M.E.F.-S and S.R. E.G., N.S. and T.R.-F. realized the sequencing and associated bioinformatics analysis. M.E.F.-S. realized RT-qPCR and all IF experiments except Myc IF in vivo and GSK3 IF, S.B. realized Myc IF in vivo and GSK3 IF and genotyping experiments and participated to the setting up of pRet IF. E.F. designed and coordinated the overall research and experiments, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.T. is co-founder and share-holder of Supersonic Imagine and J.L.G. is a consultant of Supersonic Imagine.
Extended data figures and tables
Extended Data Figure 1 Mechanical characterisation of pressure and stiffness in colonic tumours in Notch/Apc mice.
a, Crypt strain deformation analysis. Imaging of tumour initiation in wide-open colon samples. Time-dependent elliptical crypt shape change in Notch/Apc mice tissue explants analysed at 0, 2 weeks and 1 month after tamoxifen injection initiating mitotic Notch expression (Methods) (measured in n = 2 mice per time point). Notch expression is revealed by GFP. Crypt strain deformation ε = L1/((L1+L2)/2) (L1 being the smallest radius, and L2 the largest radius of the crypts elliptically deformed by the Notch dependent hyperproliferation tumour pressure induced), analysis using ImageJ. ε0week = 0.93 ± 0.05 (measured in n = 20 crypts, two mice) for control sample at 0 weeks, ε2weeks = 0.874 ± 0.06 (measured in n = 31 crypts, two mice, P = 0.029) at 2 weeks, and ε1month = 0.79 ± 0.11 (measured in n = 20 crypts, two mice, P < 0.0001), at 1 month in colon explants. Statistic test is the non-parametric exact Mann–Whitney test. Error bars are standard deviation. Scale bars, 5 μm. b, Stiffness (E) of distal colon explants embedded in agar–gelatin phantoms (Methods) measured by using shear wave elastography (see Extended Data Fig. 2e). Average values of E were obtained from at least n = 6 measurements (in two mice for 0 weeks, 1 month and 2 months, three mice for 3 months). c, In vivo stiffness images of wild-type abdomen, and of Notch/Apc tumorous colon after 3 months of tumour development (E = 70.8 ± 2.1 kPa). Elasticity measurements were taken in vivo over anaesthetized mice (average of at least n = 6 measurements, in two mice in each condition). d, Acoustic imaging distance of the magnet to the colon. The distance between the colon, observed in black after injection of echographic water gel in the lumen (Methods), and the round 3 mm diameter magnet, visualized as a result of acoustic refraction, was measured to be d = 7 ± 1 mm (measured in n = 4 mice). The white bar is 1 mm.
Extended Data Figure 2 Magnetic loading, iron oxide quantification by electronic spin resonance and strain compression calculation of magnetized wild-type and Apc+/1638N colon distal samples.
a, Magnetizing mouse colon. Subcutaneous insertion of the 3 mm diameter magnet (in red) on the back of the mice 7 mm in front of the distal colon, following UML injection in the lateral caudal vein of the mouse tail. Acoustic elastography probe in green. b, UML detection and localization in the colon. Rhodamine-labelled UML observed 1 week after injection in wild-type colon explants, in contrast to non-injected controls (measured in n = 10 images, in six mice for each condition). c, Iron oxide quantification by electronic spin resonance in wild-type mice, injected with UML or magnetic liposomes loaded with an equivalent of 120 moles and 2.2 moles of Fe(III) per mole of lipids, respectively. Iron oxide concentration in the distal colon was measured by electronic spin resonance (5% precision) from lyophilized colon explants at 1 week (magnetic liposomes: 79 ± 12 nmole g−1 (measured in n = 6 mice); UML: 183 ± 48 nmole g−1 (measured in n = 3 mice), 2 weeks (271 ± 64 nmole g−1 (measured in n = 3 mice)) and 1 month (299 ± 157 nmole g−1 (measured in n = 3 mice)) after administration. Control sample was not injected with UML and was used to set up background of iron oxide concentration. A Mann–Whitney–Wilcoxon test concluded no significant difference in iron oxide concentration during 1 month (P ≥ 0.2) in the case of the mice injected with UML. This supports the maintaining of the number and local containment of the delivered magnetic particles into the distal colon over this period of time, in agreement with the very slow biotransformation of such nanomagnets recently revealed in vivo51. The iron oxide concentration at 1 week in the distal colon of wild-type mice injected with magnetic liposomes was found around twice lower compared to samples injected with UML while the residual content tends to vanish from 2 weeks (results not shown). d, Magnetized wild-type colon samples injected with magnetic liposomes show no difference of local strain compression compared to non-injected samples or injected with magnetic liposomes without magnet (measured in n = 2 for each condition). e, Schematic representation of the ultrasound measurement setup and biomechanical imaging techniques. Left, shear wave elastography. Right, strain imaging. f, Ultrasound imaging of magnetically induced deformation of the mouse colon. Representative B-mode acoustic image (right) of a magnetically loaded colon explant and the corresponding displacement map (left) after magnet moved from 10 mm to 7 mm towards the colon (Methods). The mean value of displacement within the colon and the rest of the phantom were 35 μm and 16.9 μm, respectively (measured in n = 12 mice). g, Stiffness measurements of control (in 3 mice) and magnetized colon explants (in 6 mice) remained on the order of E = 30–35 kPa, n = 6 measurements by mice. h, Apc+/1638N mice show poor deformation at 1 week (mean elastic modulus control E = 24 ± 2 kPa, measured in n = 3 mice; UML + magnet E = 23 ± 2 kPa, measured in n = 4 mice) compared to wild type at 1 week (Fig. 1b) with similar iron oxide loading concentration of 265 ± 80 nmole g−1 (see i, n = 3) compared to the 183 ± 48 nmole g−1 of wild type (see c). Deformation decreases at 2 weeks compared to 1 week in both the Apc+/1638N (mean elastic modulus control E = 21 ± 5 kPa, measured in n = 3 mice; UML + magnet E = 33 ± 2 kPa, measured in n = 2 mice) and wild type (mean elastic modulus control E = 30 ± 8 kPa, measured in n = 3 mice; UML + magnet E = 35 ± 5 kPa, measured in n = 3 mice). i, The mean Fe(III) concentration in magnetized Apc+/1638N colon, of 258 ± 80 nmole g−1 on one month, remains on the order of magnitude of the mean concentration in wild-type colons, of 250 ± 50 nmole g−1, meaning that the magnetically induced stress remains on the same order of magnitude during 1 month in Apc+/1638N compared to wild-type mice (measured in n = 3 mice for each condition).
Extended Data Figure 3 Mechanical induction of β-catenin nuclear translocation, of target genes expression and ACF formation, though β-catenin Y654 phosphorylation in Apc+/1638N mice colon.
a, Expression levels of Myc, Axin2 and Zeb1 observed by RT–qPCR (measured in n = 8 mice for Myc, n = 9 mice for Axin2 and Zeb1). Non-significant P values between control and UML-injected conditions, P < 0.01 between UML with magnet and UML-injected and control conditions (non-parametric exact Mann–Whitney test). Error bars are standard deviation. b, Nuclear β-catenin under UML with magnet conditions, compared to controls. White spots and purple represent a positive nuclear DAPI and β-catenin co-localization, with a preference for the peripheral privileged sites of transcriptionally active chromatin (measured in n = 2 mice for each condition, ten images analysed with 4–6 crypts per image). Quantification of P values: P > 0.05 for control versus UML comparisons. P < 0.01 for comparison between UML plus magnet versus UML without magnet, by Student’s t-test. c, Number of large crypts (>1,500 μm2) in Apc+/1638N mice under UML plus magnet conditions after 1 and 2 months (all crypts mean surface 1,218.30 ± 10.64 and 1,233.8 ± 211.08 µm2, 261 crypts, measured in n = 2 mice and 195 crypts, measured in n = 3 mice, respectively), compared to control without UML (mean surface 927.6 ± 124.72 µm2, 545 crypts, measured in n = 3 mice), and in Notch/Apc mice after 1 month and 2 months (mean surfaces of 1,262.6 ± 144.35 and 1,519.5 ± 319.19 µm2, 441 and 178 crypts, respectively, measured in n = 2 mice for each condition), compared to non-tamoxifen-injected Apc+/1638N control (mean surface 927.6 ± 124.72 µm2, 545 crypts, measured in n = 3 mice). PMagnet1month < 0.01, PMagnet2months < 0.05, PNotch/Apc1month < 0.01 and PNotch/Apc2months < 0.001 (analytic Student’s t-test). Scale bars are 10 μm.
Extended Data Figure 4 Mechanically induced phospho-Tyr654-β-catenin by tumour growth pressure do not co-localize with E-cadherin but with villin.
a, Top, Ki67 expression under UML plus magnet conditions show increased number of positive cells per crypt, in contrast to controls (without UML, or without magnet with UML, measured in n = 2 mice, 33 crypts, for each condition. Blue is DAPI. Bottom, P values are of P < 0.001 between any individual conditions. Statistical test is the non-parametric exact Mann–Whitney test for all preceding quantitative experiments. b, No co-localization of pTyr654-β-catenin with sub-apical junctional E-cadherin in response to tumour growth pressure and co-localization with villin, in Apc+/1638N mice with magnetic pressure (measured in n = 2 mice by condition). c, No co-localization of pTyr654-β-catenin with sub-apical junctional E-cadherin in response to tumour growth pressure and co-localization with villin, in wild-type mice with magnetic pressure (measured in n = 2 mice by condition). d, Immunofluorescence analysis of pY1062-Ret kinase in response to ex vivo mechanical strain. The phosphorylation of Ret Y1062 is observed in ex vivo Apc+/1638N colon upon mechanical compression (Methods) (54.26 ± 16% positive crypts out of 236, measured in n = 7 mice), initiating at 1 min compression and compared to control (0.6 ± 1.3% of positive crypts out of 99, measured in n = 5 mice). Mechanical activation of pY1062-Ret is impaired by wide range PP1 and PP2 Src-family kinase inhibitors (1.55 ± 0.25% positive crypts out of 142 and 6.03 ± 3.5% positive crypts out of 192, respectively, measured in n = 2 mice for each condition). Sunitinib, a Ret inhibitor (9.85 ± 3.8% positive crypts out of 143, measured in n = 4 mice), blocks mechanical activation of pY1062-Ret and SU6656, a Src-specific inhibitor (39.5 ± 8.5% of positive crypts out of 95, measured in n = 3 mice), does not block mechanical activation of pY1062-Ret. e, Ex vivo mechanical induction of pY654-β-catenin after 20 min (measured in n = 2 mice) (upper panel), enrichment of cytoplasmic and nuclear β-catenin after 20 min (measured in n = 3 mice) (middle panel), and Myc expression after 4 h (measured in n = 6 mice) (lower panel), impaired by Sunitinib inhibitor treatment (measured in n = 2 mice by condition) in Apc+/1638N mice. β-catenin control: 13.97 ± 2.7% positive crypts out of 121, measured in n = 2 mice; compressed: 69.82 ± 8.7% positive crypts out of 119, measured in n = 2 mice; compressed + sunitinib: 24.13 ± 1.2% positive crypts out of 119, measured in n = 2 mice. Myc control: 10.96 ± 0.21% positive crypts out of 118, measured in n = 2 mice; compressed: 75.08 ± 2.8% positive crypts out of 148, measured in n = 6 mice; compressed + sunitinib: 23.36 ± 1.1% positive crypts out of 60, measured in n = 2 mice. Ex vivo mechanical compression of Apc+/1638N mice tissues caused an increase of β-catenin-positive nuclei (14.35 ± 5 a.u. (arbitrary units), measured in n = 2 mice) by a factor of 3.6 compared to the control (3.97 ± 1.9 u.a, measured in n = 2 mice), impaired in the presence of Sunitinib (4.9 ± 1.9 a.u., measured in n = 2 mice). Merged images of DAPI and β-catenin were obtained with ImageJ co-localization analysis on ten images analysed with 4–6 crypts per image. White spots represent a positive co-localization. f, No co-localization of pTyr654-β-catenin with sub-apical junctional E-cadherin and co-localization with villin in Notch/Apc mice (measured in n = 2 mice). Here, the original colour for E-cadherin and villin was blue to avoid Notch–GFP green fluorescence, and was changed to green for the colour coherence of the complete figure. g, No co-localization of pTyr654-β-catenin with sub-apical junctional E-cadherin and co-localization with villin, in Apc+/1638N ex vivo compressed mice. Some apical E-cadherin is observed (measured in n = 2 mice).
Extended Data Figure 5 Induction of ACF and adenoma formation in response to magnetic pressure mimicking tumour growth in vivo observed by colonoscopy in Apc+/1638N mice.
a, Apc+/1638N control: time 0, 0 aberrant crypt; t = 5 days, 0 aberrant crypt; t = 15 days, 2 ± 1 ACF; t = 1 month, 4 ± 1 ACF; t = 1.5 months, 4 ± 1 ACF; t = 2 months, 5 ± 2 ACF; t = 2.5 months, 3 ± 2 ACF (measured in n = 3 mice). Apc+/1638N UML: time 0, 0 aberrant crypt; t = 5 days, 0 aberrant crypt; t = 15 days, 2 ± 1 ACF; t = 1 month, 4 ± 1 ACF; t = 1.5 months, 4 ± 3 ACF; t = 2 months, 4 ± 2 ACF; t = 2.5 months, 3 ± 1 ACF (measured in n = 3 mice). Mean size of ACF remained constant and around 874.5 ± 414 μm2 in both cases. Apc+/1638N UML + magnet: time 0, 0 aberrant crypt; t = 5 days, 2 ± 1 ACF of size 1,146.9 ± 101 μm2; t = 15 days, 8 ± 2 ACF of size 1,777.7 ± 775 μm2 plus one small adenoma of 4,229.38 μm2 size; t = 1 month, 8 ± 2 ACF of mean size 3,483.9 ± 665 μm2 plus one small adenoma; t = 1.5 months, 8 ± 2 ACF plus one bigger adenoma of 6,379.92 μm2 size; t = 2 months, 8 ± 2 ACF plus one small adenoma of 3,512.54 μm2 size plus one bigger adenoma; t = 2.5 months, 8 ± 2 ACF plus one small adenoma plus one bigger adenoma of 6,523.28 μm2 size (measured in n = 3 mice). Time 0 corresponds to 4-month-old animals. b, Histologic characterization of the adenoma carcinoma induced by magnetic mechanical pressure mimicking tumour growth in the Apc+/1638N mice using haematoxylin and eosin stainning. Apc+/1638N UML 2.5 months, no ademona carcinoma is observed (measured in n = 3 mice); Apc+/1638N UML magnet 2.5 months and zoom, the crypt fusion and loss of apico-basal polarity in nucleus position show the carcinoma nature of the two adenoma observed (measured in n = 3 mice). All mice were injected at 4 months. c, LOH (loss of heterozygosity) evaluation in colonic tissues of Apc+/1638N mice under UML mechanical pressure by genotyping at 15 days. PCR analysis of DNA derived from Apc+/1638N mice with oligonucleotides detecting the wild-type and mutant Apc1638N allele. Wild-type mice show no Apc1638N allele (measured in n = 3 mice), whereas Apc+/1638N heterozygous mice show a ratio Apc:Apc1638N of 1.1 ± 0.1 (measured in n = 3 mice). Apc+/1638N mice with UML and magnet show a ratio Apc:Apc1638N of 1.1 ± 0.1 (measured in n = 2 mice).
Extended Data Figure 6 Mechanical activation of Ret in both Apc+/1638N and wild-type mice.
a, Screening of the mechanical activation of the Src family kinases known to phosphorylate β-catenin Tyr654 and to be mechanosensitive in cell culture. Ret activation by Ret Tyr1062 phosphorylation under uni-axial compression (see Extended Data Fig. 4d legend for statistics). No phosphorylation of the Tyr568/570 site of activation of Kit under uni-axial compression. Control samples showed 6.3 ± 1.4% positive crypts (258 total crypts observed, measured in n = 4 mice) and compressed samples 6.3 ± 1.2% positive crypts (399 total crypts observed, measured in n = 6 mice). No phosphorylation of the Tyr1238/1239 site of activation of Ron under uni-axial compression. All negative crypts both in compressed samples (306 total crypts observed, measured in n = 4 mice) and controls (347 total crypts observed, measured in n = 4 mice). No phosphorylation of the Tyr537 site of activation of Yes. Control samples showed 20.8 ± 1.3% positive crypts (307 total crypts observed, measured in n = 4 mice) and compressed samples showed 15.7 ± 1.9% positive crypts (312 total crypts observed, measured in n = 4 mice). Positive crypts had only one positive stained cell. No significant phosphorylation increase of the Tyr209/211 site of activation of Hck under uni-axial compression (measured in n = 4 mice for each condition). 89.2 ± 6.6% positive crypts in compressed samples (268 total crypts observed, measured in n = 4 mice) compared to control 88.3 ± 2.6% positive crypts (258 total crypts observed, measured in n = 4 mice). No phosphorylation of the Tyr412 site of activation of Abl under uni-axial compression. All negative crypts both in compressed samples (326 total crypts observed, measured in n = 4 mice) and controls (332 total crypts observed, measured in n = 4 mice). b, Inhibition of pTyr1062- Ret, pTyr654-β-catenin and Myc mechanical induction by the two additional inhibitors of Ret, vandetanib and ponatinib. Top, Tyr1062 phosphorylation of Ret under uni-axial compression (54.16 ± 15% positive crypts of 236, n = 7 mice), initiating at 1-min compression, compared to control (0.6 ± 0.8% positive crypts of 99, measured in n = 5 mice) is inhibited in the presence of vandetanib (7.91 ± 1.2% positive crypts of 128 total crypts observed (measured in n = 2 mice)) and ponatinib (5.17 ± 1.3% positive crypts out of 193 total crypts observed (measured in n = 2 mice)). Middle, initiation of the β-catenin oncogenic pathway by phosphorylation of β-catenin on Tyr654 under uni-axial compression (69.82 ± 8.7% positive crypts of 119, measured in n = 2 mice) compared to control (13.97 ± 2.7% positive crypts of 121, measured in n = 2 mice) is inhibited in the presence of vandetanib (5.89 ± 4.2% positive crypts of 102 total crypts observed, measured in n = 2 mice) and ponatinib (9.13 ± 3.3% positive crypts of 120 total crypts observed, measured in n = 2 mice). Bottom, mechanical induction of Myc under uni-axial compression (50.5 ± 0.2% positive crypts of 146, measured in n = 2 mice) compared to control (11 ± 0.2% positive crypts of 118, measured in n = 2 mice), is altered in the presence of vandetanib (36.7 ± 2.2% positive crypts of 108 total crypts observed, measured in n = 2 mice) and ponatinib (20.2 ± 3.5% positive crypts of 182 total crypts observed, measured in n = 2 mice). The concentrations used were 10 μM for vandetanib, and 100 nM for Ponatinib. c, pY1062-Ret induction quantified by western blot. Control (measured in n = 3 mice), UML + magnet (measured in n = 3 mice) and UML alone (measured in n = 3 mice) conditions. P > 0.05 between control and UML, P < 0.01 between UML and UML + magnet, Student t-test. d, Immunofluorescence analysis of wild-type and UML-injected colon in the presence of the magnet of pY1062-Ret (at 2 weeks; control: measured in n = 3 mice, 300 crypts minimum; UML + magnet: measured in n = 3 mice, 469 crypts), of pY654-β-catenin apically (at 1 month; control: measured in n = 3 mice, 798 crypts; UML + magnet: measured in n = 2 mice, 495 crypts), of cytoplasmic and nuclear enrichment of β-catenin (at 2 months; control: 14 images analysed with 4–6 crypts per image; UML + magnet: ten images analysed with 4–6 crypts per image; measured in n = 2 mice for each condition), and of the expression of Myc (at 2 months; control: measured in n = 3 mice, 245 crypts; UML + magnet: measured in n = 4 mice, 453 crypts). White spots and purple represent positive co-localization, with a preference for peripheral sites. e, Quantification of d. P values: pRet (P < 0.001), pβ-catenin (P < 0.001), β-cat (P < 0.001) and Myc (P < 0.001), by Student’s t-test. f, Percentage of crypts exceeding 1,500 µm2 of mean surface area 3 months after UML injection mimicking tumour growth pressure (mean surface 1,311.29 ± 82 µm2, measured in n = 2 mice, 285 crypts) compared to the control without UML (mean surface 914.3 ± 189.09 µm2, measured in n = 3 mice, 432 crypts). PMagnet3month < 0.05 (analytic Student’s t- test). Scale bars are 10 μm.
Extended Data Figure 7 Ret activation is rapidly and non-cell autonomously induced by the mechanical strains developed by magnetic pressure.
a, Rapid mechanical activation of pTyr1062-Ret initiating 30 min after UML injection in the presence of magnet in wild-type and in Apc+/1638N mice. Wild-type control: all negative crypts. Wild type + UML + magnet 30 min: 20.2 ± 0.56% positive crypts (measured in n = 2 mice, 372 crypts), 1 h: 44.56 ± 22.81% positive crypts (measured in n = 2 mice, 377 crypts), 2 h: 61.4 ± 24.12% positive crypts (measured in n = 2 mice, 458 crypts), 4 h: 67.5 ± 10.23% positive crypts (measured in n = 2 mice, 510 crypts). Apc+/1638N control: 1.22 ± 0.48% positive crypts (measured in n = 4 mice, 556 crypts). Apc+/1638N + UML + magnet 30 min: 20.18 ± 3.73% positive crypts (measured in n = 2 mice, 417 crypts), 1 h: 28.77 ± 7.87% positive crypts (measured in n = 2 mice, 382 crypts). b, Maintenance of mechanical activation of Tyr1062-Ret 24 h after UML injection in the presence of magnet in Apc+/1638N mice. Apc+/1638N control: 1.22 ± 0.5% pTyr1062-Ret-positive crypts out of 556 total crypts (measured in n = 4 mice). Apc+/1638N + UML (without magnet): 3.88 ± 0.6% of pTyr1062-Ret-positive crypts out of 568 total crypts (measured in n = 2 mice). Apc+/1638N + UML + magnet: 14.15 ± 2.5% of pTyr1062-Ret-positive crypts out of 499 total crypts (measured in n = 2 mice). c, Mechanical activation of pTyr1062-Ret kinase in non-UML-loaded local domains, after UML injection and magnet implantation. A strong phosphorylation of Ret Tyr1062 (green) was observed not only in the domains where UML were accumulated (not shown) but also in UML-absent domains (negative signal for fluorescent rhodamine, left) in Apc+/1638N colons (measured in n = 4 mice for each condition). d, No mechanical activation of pTyr1062-Ret kinase after UML injection in the absence of magnet in Apc-deficient colons. In some domains where an accumulation of UML in the conjunctive tissue could be observed (left) no Ret phosphorylation was observed (right) (measured in n = 3 mice for each condition). e, Expression of GDNF, artemin, and neurturin do not change in magnetized Apc+/1638N colon explants. We observed no effect of magnetization of the tissue in the expression of any ligand of Ret as compared to the control not injected with UML, to the non-magnetized colon tissue injected with UML and to the wild-type kidney positive control expressing the three ligands of Ret (minimum of 300 crypts analysed, measured in n = 2 mice for each condition). f, Mechanical inactivation of GSK-3β through Ser9 phosphorylation and mechanical activation of the upstream Akt through Ser473 phosphorylation in magnetized Apc-deficient colon explants. Increased phosphorylation of GSK-3β Ser9 was observed in the magnetized tissue at 2 weeks (33.8 ± 0.4% of 307 crypts), compared to the non-magnetized control (12.7 ± 5.15% of 560 crypts) and to the UML-injected colon sample without magnet (7.24 ± 2% of 138 crypts) (measured in n = 2 mice for each condition). Increased phosphorylation of Akt Ser473 was observed in the magnetized tissue at 2 weeks (40.6 ± 4.5% of 378 crypts, measured in n = 3 mice), compared to the non-magnetized control (3.2 ± 1.1% of 596 crypts, measured in n = 3 mice) and to the UML-injected colon sample without magnet (2.7 ± 0.2% of 225 crypts, measured in n = 2 mice). g, Ret-dependent mechanical inactivation of GSK-3β and activation of the upstream Akt after ex vivo global compression of Apc-deficient colon explants. GSK-3β: control, 12.5 ± 5.2% positive crypts out of 320 (measured in n = 2 mice); compressed, 43.26 ± 11% positive crypts out of 784 (measured in n = 2 mice); compressed + sunitinib, 19.7 ± 12% positive crypts out of 216 (measured in n = 2 mice); compressed + vandetanib, 14.4 ± 0.1% positive crypts out of 114 (measured in n = 2 mice); compressed + ponatinib, 15.45 ± 5.6% positive crypts out of 230 (measured in n = 2 mice for each condition). Akt: control, 5.3 ± 4.3% positive crypts out of 519; compressed, 46.6 ± 3.5% positive crypts out of 554; compressed + sunitinib, 14.26 ± 8.3% positive crypts out of 560; compressed + vandetanib, 5.37 ± 0.5% positive crypts out of 571; compressed + ponatinib, 7.52 ± 0.8% positive crypts out of 443 (measured in n = 2 mice for each condition).
Extended Data Figure 8 Notch/Apc colon shows an activation of the Ret/β-catenin/Myc mechanotransductive signalling pathway one month after tumour growth initiation.
a, Top, Ret Y1062 phosphorylation (measured in n = 4 mice, 317 crypts), apical β-catenin Y654 phosphorylation (measured in n = 4 mice, 223 crypts), cytoplasmic and nuclear β-catenin enrichment (measured in n = 2 mice, 16 images analysed with 4–6 crypts per image), and Myc expression activation (at 2.5 months, measured in n = 2 mice, 194 crypts), in Notch/Apc tumorous tissue compared to non-tamoxifen-injected Notch/Apc mice controls (measured in n = 4 mice, 264 crypts; measured in n = 4 mice, 198 crypts; measured in n = 2 mice, 18 images analysed with 4–6 crypts per image; measured in n = 2 mice, 315 crypts, respectively). GFP fluorescence (green) reveals a nuclear overexpression of Notch in the tumorous crypts. Note that at 1 month after tamoxifen injection, GFP expression is often diffuse and found in the nuclei and cytoplasm. Immunofluorescence staining and ImageJ co-localization analysis revealed an enrichment of nuclear β-catenin (white and purple spots represent a positive co-localization between β-catenin (red) and DAPI (blue)) by a factor of 5.8 in Notch/Apc colon samples 1 month after tamoxifen injection (11.64 ± 2.5 a.u., measured in n = 2 mice), compared to the control (1.99 ± 0.8 a.u., measured in n = 2 mice). Bottom, phosphorylation of β-catenin Y654 in Notch-negative crypts in tamoxifen-injected crypts (measured in n = 4 mice, 68 crypts, yellow arrows) compared to non-tamoxifen-injected control conditions (measured in n = 4 mice, 198 crypts). Cytoplasmic enrichment and nuclear translocation of β-catenin in Notch-negative crypts (measured in n = 4 mice). Nuclear translocation of β-catenin is assessed by co-localization with DAPI (in white) in Notch-negative crypts of Notch/Apc tissues after 1 month of tamoxifen injection compared to non-tamoxifen-injected control conditions. White spots and purple represent a positive nuclear DAPI and β-catenin co-localization, with a preference for the peripheral privileged sites of transcriptionally active chromatin. Immunofluorescence staining and ImageJ co-localization analysis revealed an enrichment of nuclear β-catenin (white and purple spots represent a positive co-localization between β-catenin (red) and DAPI (blue)) by a factor of 15 in Notch-negative crypts in colon samples 1 month after tamoxifen injection (33.6 ± 8.7 a.u., measured in n = 2 mice), compared to the control (1.99 ± 0.8 a.u., measured in n = 2 mice). Ten images with 4–6 crypts per image were analysed in each condition. Scale bars are 10 μm. b, Quantification of a. P < 0.001 in all cases, Student’s t-test.
Extended Data Figure 9 Ret activation is non-cell autonomously induced by the mechanical strains developed by tumour pressure in Notch/Apc mice.
a, Early mechanical activation of pTyr1062-Ret kinase in crypts totally surrounded by a Notch-negative domain in tumorous Notch/Apc colon explants. Notch/Apc mice were injected with tamoxifen for four consecutive days (instead of 5 days, see Methods) to induce tumour growth initiation, and colon explants were analysed on the fifth day by immunofluorescence. Ret phosphorylation was activated in 12.7 ± 2.3% of Notch-negative crypts (22 pTyr1062-Ret-positive crypts of a total of 177 GFP-negative crypts) completely surrounded by Notch-negative crypts (GFP-negative), measured in n = 4 mice. b, No phosphorylation of Ret Tyr1062 in Notch-overexpressing cells in early tumorous Notch/Apc colon explants. 34.9 ± 8.8% of GFP-positive crypts (yellow arrows) showed no expression of pTyr1062-Ret (39 crypts out of a total of 113 crypts, measured in n = 4 mice). c, Strain deformation of Notch/Apc 4 days after tamoxifen injection. ε4d = 0.89 ± 0.08 (measured in n = 169 crypts, in three mice), ε0d = 0.93 ± 0.05 (measured in n = 20 crypts in two mice), P = 0.004 (Mann–Whitney exact test), leading to a mean tumour stress of S4d = 0.9 ± 0.1 kPa (following Hooke’s law S = E × ε with ENotch/Apc = 22.8 ± 4.8 kPa (see Extended Data Fig. 1b)). Error bars are s.d. d, Expression of GDNF, artemin and neurturin Ret ligands does not change in tumour-initiated Notch/Apc colon. We observed no effect of tumour growth in the expression of any of the ligands (red) as compared to the control Notch/Apc not injected with tamoxifen, and to the wild-type kidney positive controls in which the three ligands of Ret are expressed. GFP fluorescence reveals positive expression of Notch and labels the tumorous crypts (minimum 300 crypts analysed, measured in n = 2 mice for each condition). At 1 month after tamoxifen (tam.) injection, GFP expression is often diffuse and found in the nuclei and cytoplasm.
Extended Data Figure 10 Summary of the results.
a, Magnetically induced tumour growth pressure. b, Notch-induced tumour growth pressure. c, Ret/β-catenin promote tumorigenic target gene expression and ACF formation and are mechanically induced by tumour growth pressure.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data 1-11, Supplementary Discussions 1-3 and additional references. (PDF 669 kb)
Rights and permissions
About this article
Cite this article
Fernández-Sánchez, M., Barbier, S., Whitehead, J. et al. Mechanical induction of the tumorigenic β-catenin pathway by tumour growth pressure. Nature 523, 92–95 (2015). https://doi.org/10.1038/nature14329
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14329
This article is cited by
-
Intravital measurements of solid stresses in tumours reveal length-scale and microenvironmentally dependent force transmission
Nature Biomedical Engineering (2023)
-
Biophysics in tumor growth and progression: from single mechano-sensitive molecules to mechanomedicine
Oncogene (2023)
-
Cancer-associated fibroblasts actively compress cancer cells and modulate mechanotransduction
Nature Communications (2023)
-
Field carcinogenesis and biological significance of the potential of the bystander effect: carcinogenesis, therapeutic response, and tissue regeneration
Surgery Today (2023)
-
Peristalsis-Associated Mechanotransduction Drives Malignant Progression of Colorectal Cancer
Cellular and Molecular Bioengineering (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.