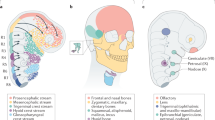
Abstract
It has been more than 30 years since the publication of the new head hypothesis, which proposed that the vertebrate head is an evolutionary novelty resulting from the emergence of neural crest and cranial placodes. Neural crest generates the skull and associated connective tissues, whereas placodes produce sensory organs. However, neither crest nor placodes produce head muscles, which are a crucial component of the complex vertebrate head. We discuss emerging evidence for a surprising link between the evolution of head muscles and chambered hearts — both systems arise from a common pool of mesoderm progenitor cells within the cardiopharyngeal field of vertebrate embryos. We consider the origin of this field in non-vertebrate chordates and its evolution in vertebrates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gans, C. & Northcutt, R. G. Neural crest and the origin of vertebrates: a new head. Science 220, 268–273 (1983). This highly influential paper argued that the evolution of head structures derived from neural crest and cranial placodes had a crucial role in the transition to early vertebrates.
Patthey, C., Schlosser, G. & Shimeld, S. M. The evolutionary history of vertebrate cranial placodes — I: cell type evolution. Dev. Biol. 389, 82–97 (2014).
Graham, A. & Shimeld, S. M. The origin and evolution of the ectodermal placodes. J. Anat. 222, 32–40 (2013).
Northcutt, R. G. The new head hypothesis revisited. J. Exp. Zool. B Mol. Dev. Evol. 304B, 274–297 (2005).
Kuratani, S. Evolution. A muscular perspective on vertebrate evolution. Science 341, 139–140 (2013).
Trinajstic, K. et al. Fossil musculature of the most primitive jawed vertebrates. Science 341, 160–164 (2013).
Meilhac, S. M., Esner, M., Kelly, R. G., Nicolas, J. F. & Buckingham, M. E. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev. Cell 6, 685–698 (2004).
Kelly, R. G. The second heart field. Curr. Top. Dev. Biol. 100, 33–65 (2012).
Tzahor, E. & Evans, S. M. Pharyngeal mesoderm development during embryogenesis: implications for both heart and head myogenesis. Cardiovasc. Res. 91, 196–202 (2011).
Kelly, R. G., Brown, N. A. & Buckingham, M. E. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev. Cell 1, 435–440 (2001). Discovery of the mammalian SHF, demonstrating that myocardium at the arterial pole of the heart originates in adjacent pharyngeal mesoderm.
Mjaatvedt, C. H. et al. The outflow tract of the heart is recruited from a novel heart-forming field. Dev. Biol. 238, 97–109 (2001).
Waldo, K. L. et al. Conotruncal myocardium arises from a secondary heart field. Development 128, 3179–3188 (2001).
Nathan, E. et al. The contribution of Islet1-expressing splanchnic mesoderm cells to distinct branchiomeric muscles reveals significant heterogeneity in head muscle development. Development 135, 647–657 (2008). This article provides a definition of the contribution of pharyngeal mesoderm to branchiomeric muscles in both chick and mouse embryos.
Mesbah, K. et al. Identification of a Tbx1/Tbx2/Tbx3 genetic pathway governing pharyngeal and arterial pole morphogenesis. Hum. Mol. Genet. 21, 1217–1229 (2012).
Tirosh-Finkel, L., Elhanany, H., Rinon, A. & Tzahor, E. Mesoderm progenitor cells of common origin contribute to the head musculature and the cardiac outflow tract. Development 133, 1943–1953 (2006). This article demonstrates, using fate-mapping and experimental manipulation in the avian embryo, that cranial mesoderm gives rise both to head muscles and outflow tract myocardium.
Tzahor, E. & Lassar, A. B. Wnt signals from the neural tube block ectopic cardiogenesis. Genes Dev. 15, 255–260 (2001).
Noden, D. M. & Trainor, P. A. Relations and interactions between cranial mesoderm and neural crest populations. J. Anat. 207, 575–601 (2005).
Hutson, M. R. & Kirby, M. L. Neural crest and cardiovascular development: a 20-year perspective. Birth Defects Res. C Embryo Today 69, 2–13 (2003).
Rinon, A. et al. Cranial neural crest cells regulate head muscle patterning and differentiation during vertebrate embryogenesis. Development 134, 3065–3075 (2007).
Bothe, I. & Dietrich, S. The molecular setup of the avian head mesoderm and its implication for craniofacial myogenesis. Dev. Dynam. 235, 2845–2860 (2006).
Grifone, R. & Kelly, R. G. Heartening news for head muscle development. Trends Genet. 23, 365–369 (2007).
Sambasivan, R., Kuratani, S. & Tajbakhsh, S. An eye on the head: the development and evolution of craniofacial muscles. Development 138, 2401–2415 (2011).
Cai, C. L. et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell 5, 877–889 (2003).
Harel, I. et al. Distinct origins and genetic programs of head muscle satellite cells. Dev. Cell 16, 822–832 (2009). This article demonstrates the diversity of lineages constituting craniofacial skeletal muscles and their associated satellite cells using a series of Cre lines to genetically trace trunk and cranial myogenic progenitor cells, leading to an Isl1-lineage-based definition of CPF-derived craniofacial muscles.
Dodou, E., Verzi, M. P., Anderson, J. P., Xu, S. M. & Black, B. L. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development 131, 3931–3942 (2004).
Watanabe, Y. et al. Fibroblast growth factor 10 gene regulation in the second heart field by Tbx1, Nkx2–5, and Islet1 reveals a genetic switch for down-regulation in the myocardium. Proc. Natl Acad. Sci. USA 109, 18273–18280 (2012).
Prall, O. W. et al. An Nkx2–5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell 128, 947–959 (2007).
Scambler, P. J. 22q11 deletion syndrome: a role for TBX1 in pharyngeal and cardiovascular development. Pediatr. Cardiol. 31, 378–390 (2010).
Liao, J. et al. Identification of downstream genetic pathways of Tbx1 in the second heart field. Dev. Biol. 316, 524–537 (2008).
Chen, L., Fulcoli, F. G., Tang, S. & Baldini, A. Tbx1 regulates proliferation and differentiation of multipotent heart progenitors. Circ. Res. 105, 842–851 (2009).
Hami, D., Grimes, A. C., Tsai, H. J. & Kirby, M. L. Zebrafish cardiac development requires a conserved secondary heart field. Development 138, 2389–2398 (2011).
Kelly, R. G., Jerome-Majewska, L. A. & Papaioannou, V. E. The del22q11.2 candidate gene Tbx1 regulates branchiomeric myogenesis. Hum. Mol. Genet. 13, 2829–2840 (2004). This paper reports the genetic identification of Tbx1 as a regulator of craniofacial myogenesis in mice, supporting the existence of distinct upstream regulatory hierarchies controlling head and trunk myogenesis.
Kong, P. et al. Tbx1 is required autonomously for cell survival and fate in the pharyngeal core mesoderm to form the muscles of mastication. Hum. Mol. Genet. 23, 4215–4231 (2014).
Castellanos, R., Xie, Q., Zheng, D., Cvekl, A. & Morrow, B. E. Mammalian TBX1 preferentially binds and regulates downstream targets via a tandem T-site repeat. PLoS ONE 9, e95151 (2014).
Harel, I. et al. Pharyngeal mesoderm regulatory network controls cardiac and head muscle morphogenesis. Proc. Natl Acad. Sci. USA 109, 18839–18844 (2012).
Lescroart, F. et al. Clonal analysis reveals common lineage relationships between head muscles and second heart field derivatives in the mouse embryo. Development 137, 3269–3279 (2010). This retrospective lineage analysis provides evidence for the existence of common progenitor cells in the mouse embryo that give rise to myocardium of the right ventricle and first-arch-derived muscles, and to the arterial pole of the heart and second-arch-derived muscles.
Romer, A. S. & Parson, T. S. The Vertebrate Body (Saunder's College Publishing, 1977).
Diogo, R. & Abdala, V. Muscles of Vertebrates: Comparative Anatomy, Evolution, Homologies and Development (CRC, 2010). This monograph provides an overview on the comparative anatomy, evolution and homologies of the head and limb muscles in all major extant vertebrate groups with special focus on the developmental and evolutionary history of the muscles of Homo sapiens.
Devine, W. P., Wythe, J. D., George, M., Koshiba-Takeuchi, K. & Bruneau, B. G. Early patterning and specification of cardiac progenitors in gastrulating mesoderm. eLife 3, e03848 (2014).
Lescroart, F. et al. Early lineage restriction in temporally distinct populations of Mesp1 progenitors during mammalian heart development. Nature Cell Biol. 16, 829–840 (2014).
Olson, E. N. Gene regulatory networks in the evolution and development of the heart. Science 313, 1922–1927 (2006).
Tzahor, E. Heart and craniofacial muscle development: a new developmental theme of distinct myogenic fields. Dev. Biol. 327, 273–279 (2009).
Diogo, R. & Wood, B. A. Comparative Anatomy and Phylogeny of Primate Muscles and Human Evolution (CRC, 2012).
Wachtler, F. & Jacob, M. Origin and development of the cranial skeletal muscles. Bibl. Anat. 1986, 24–46 (1986).
Noden, D. M. The embryonic origins of avian cephalic and cervical muscles and associated connective tissues. Am. J. Anat. 168, 257–276 (1983).
Noden, D. M. & Francis-West, P. The differentiation and morphogenesis of craniofacial muscles. Dev. Dynam. 235, 1194–1218 (2006).
Diogo, R., Hinits, Y. & Hughes, S. M. Development of mandibular, hyoid and hypobranchial muscles in the zebrafish: homologies and evolution of these muscles within bony fishes and tetrapods. BMC Dev. Biol. 8, 24 (2008).
Diogo, R., Abdala, V., Lonergan, N. & Wood, B. A. From fish to modern humans — comparative anatomy, homologies and evolution of the head and neck musculature. J. Anat. 213, 391–424 (2008).
Kuraku, S., Hoshiyama, D., Katoh, K., Suga, H. & Miyata, T. Monophyly of lampreys and hagfishes supported by nuclear DNA-coded genes. J. Mol. Evol. 49, 729–735 (1999).
Delarbre, C., Gallut, C., Barriel, V., Janvier, P. & Gachelin, G. Complete mitochondrial DNA of the hagfish, Eptatretus burgeri: the comparative analysis of mitochondrial DNA sequences strongly supports the cyclostome monophyly. Mol. Phylogenet. Evol. 22, 184–192 (2002).
Delarbre, C. et al. The complete nucleotide sequence of the mitochondrial DNA of the agnathan Lampetra fluviatilis: bearings on the phylogeny of cyclostomes. Mol. Biol. Evol. 17, 519–529 (2000).
Heimberg, A. M., Cowper-Sal-lari, R., Semon, M., Donoghue, P. C. & Peterson, K. J. microRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate. Proc. Natl Acad. Sci. USA 107, 19379–19383 (2010).
Ziermann, J. M., Miyashita, T. & Diogo, R. Cephalic muscles of Cyclostomes (hagfishes and lampreys) and Chondrichthyes (sharks, rays and holocephalans): comparative anatomy and early evolution of the vertebrate head. Zool. J. Linn. Soc. 172, 771–802 (2014).
Adachi, N. & Kuratani, S. Development of head and trunk mesoderm in the dogfish, Scyliorhinus torazame: I. Embryology and morphology of the head cavities and related structures. Evol. Dev. 14, 234–256 (2012).
Adachi, N., Takechi, M., Hirai, T. & Kuratani, S. Development of the head and trunk mesoderm in the dogfish, Scyliorhinus torazame: II. Comparison of gene expression between the head mesoderm and somites with reference to the origin of the vertebrate head. Evol. Dev. 14, 257–276 (2012).
Kuratani, S., Adachi, N., Wada, N., Oisi, Y. & Sugahara, F. Developmental and evolutionary significance of the mandibular arch and prechordal/premandibular cranium in vertebrates: revising the heterotopy scenario of gnathostome jaw evolution. J. Anat. 222, 41–55 (2013).
Kusakabe, R., Kuraku, S. & Kuratani, S. Expression and interaction of muscle-related genes in the lamprey imply the evolutionary scenario for vertebrate skeletal muscle, in association with the acquisition of the neck and fins. Dev. Biol. 350, 217–227 (2011).
Kokubo, N. et al. Mechanisms of heart development in the Japanese lamprey, Lethenteron japonicum. Evol. Dev. 12, 34–44 (2010).
Onimaru, K., Shoguchi, E., Kuratani, S. & Tanaka, M. Development and evolution of the lateral plate mesoderm: comparative analysis of amphioxus and lamprey with implications for the acquisition of paired fins. Dev. Biol. 359, 124–136 (2011).
Sauka-Spengler, T., Le Mentec, C., Lepage, M. & Mazan, S. Embryonic expression of Tbx1, a DiGeorge syndrome candidate gene, in the lamprey Lampetra fluviatilis. Gene Expr. Patterns 2, 99–103 (2002).
Tiecke, E. et al. Identification and developmental expression of two Tbx1/10-related genes in the agnathan Lethenteron japonicum. Dev. Genes Evol. 217, 691–697 (2007).
Simões-Costa, M. S. et al. The evolutionary origin of cardiac chambers. Dev. Biol. 277, 1–15 (2005).
Moorman, A. F. & Christoffels, V. M. Cardiac chamber formation: development, genes, and evolution. Physiol. Rev. 83, 1223–1267 (2003).
Ziermann, J. M. & Diogo, R. Cranial muscle development in the model organism Ambystoma mexicanum: implications for tetrapod and vertebrate comparative and evolutionary morphology and notes on ontogeny and phylogeny. Anat. Rec. (Hoboken) 296, 1031–1048 (2013).
Matsuoka, T. et al. Neural crest origins of the neck and shoulder. Nature 436, 347–355 (2005).
Ziermann, J. M. & Diogo, R. Cranial muscle development in frogs with different developmental modes: direct development versus biphasic development. J. Morphol. 275, 398–413 (2014).
Shearman, R. M. & Burke, A. C. The lateral somitic frontier in ontogeny and phylogeny. J. Exp. Zool. B Mol. Dev. Evol. 312, 603–612 (2009).
Minchin, J. E. et al. Oesophageal and sternohyal muscle fibres are novel Pax3-dependent migratory somite derivatives essential for ingestion. Development 140, 2972–2984 (2013).
Abdala, V. & Diogo, R. Comparative anatomy, homologies and evolution of the pectoral and forelimb musculature of tetrapods with special attention to extant limbed amphibians and reptiles. J. Anat. 217, 536–573 (2010).
Edgeworth, F. H. The Cranial Muscles of Vertebrates (The University Press, Cambridge 1935). This 80-year-old publication continues to be the most complete compendium on the anatomical development of the head muscles of vertebrates.
Piotrowski, T. & Nusslein-Volhard, C. The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (Danio rerio). Dev. Biol. 225, 339–356 (2000).
Noden, D. M. & Schneider, R. A. Neural crest cells and the community of plan for craniofacial development: historical debates and current perspectives. Adv. Exp. Med. Biol. 589, 1–23 (2006).
Theis, S. et al. The occipital lateral plate mesoderm is a novel source for vertebrate neck musculature. Development 137, 2961–2971 (2010).
Gegenbaur, C. Elements of Comparative Anatomy (Macmillan, 1878).
Gillis, J. A., Dahn, R. D. & Shubin, N. H. Shared developmental mechanisms pattern the vertebrate gill arch and paired fin skeletons. Proc. Natl Acad. Sci. USA 106, 5720–5724 (2009).
Putnam, N. H. et al. The amphioxus genome and the evolution of the chordate karyotype. Nature 453, 1064–1071 (2008).
Delsuc, F., Brinkmann, H., Chourrout, D. & Philippe, H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 439, 965–968 (2006).
Butler, A. B. The serial transformation hypothesis of vertebrate origins: comment on “The new head hypothesis revisited”. J. Exp. Zool. B Mol. Dev. Evol. 306, 419–424 (2006).
Gans, C. Stages in the origin of vertebrates: analysis by means of scenarios. Biol. Rev. Camb. Philos. Soc. 64, 221–268 (1989).
Mazet, F. et al. Molecular evidence from Ciona intestinalis for the evolutionary origin of vertebrate sensory placodes. Dev. Biol. 282, 494–508 (2005).
Mazet, F. & Shimeld, S. M. Molecular evidence from ascidians for the evolutionary origin of vertebrate cranial sensory placodes. J. Exp. Zool. B Mol. Dev. Evol. 304, 340–346 (2005).
Wagner, E. & Levine, M. FGF signaling establishes the anterior border of the Ciona neural tube. Development 139, 2351–2359 (2012).
Christiaen, L., Bourrat, F. & Joly, J. S. A modular cis-regulatory system controls isoform-specific pitx expression in ascidian stomodaeum. Dev. Biol. 277, 557–566 (2005).
Christiaen, L. et al. Pitx genes in Tunicates provide new molecular insight into the evolutionary origin of pituitary. Gene 287, 107–113 (2002).
Abitua, P. B., Wagner, E., Navarrete, I. A. & Levine, M. Identification of a rudimentary neural crest in a non-vertebrate chordate. Nature 492, 104–107 (2012).
Satou, Y., Imai, K. S. & Satoh, N. The ascidian Mesp gene specifies heart precursor cells. Development 131, 2533–2541 (2004).
Davidson, B., Shi, W. & Levine, M. Uncoupling heart cell specification and migration in the simple chordate Ciona intestinalis. Development 132, 4811–4818 (2005).
Christiaen, L. et al. The transcription/migration interface in heart precursors of Ciona intestinalis. Science 320, 1349–1352 (2008).
Davidson, B., Shi, W., Beh, J., Christiaen, L. & Levine, M. FGF signaling delineates the cardiac progenitor field in the simple chordate, Ciona intestinalis. Genes Dev. 20, 2728–2738 (2006).
Beh, J., Shi, W., Levine, M., Davidson, B. & Christiaen, L. FoxF is essential for FGF-induced migration of heart progenitor cells in the ascidian Ciona intestinalis. Development 134, 3297–3305 (2007).
Christiaen, L., Stolfi, A. & Levine, M. BMP signaling coordinates gene expression and cell migration during precardiac mesoderm development. Dev. Biol. 340, 179–187 (2010).
Ragkousi, K., Beh, J., Sweeney, S., Starobinska, E. & Davidson, B. A single GATA factor plays discrete, lineage specific roles in ascidian heart development. Dev. Biol. 352, 154–163 (2011).
Stolfi, A. et al. Early chordate origins of the vertebrate second heart field. Science 329, 565–568 (2010). This article reports the discovery of the CPF in C. intestinalis using dynamic imaging and genetics, revealing striking genetic similarities with vertebrate pharyngeal mesoderm giving rise to head muscles and SHF-derived parts of the heart.
Tolkin, T. & Christiaen, L. Development and evolution of the ascidian cardiogenic mesoderm. Curr. Top. Dev. Biol. 100, 107–142 (2012).
Wang, W., Razy-Krajka, F., Siu, E., Ketcham, A. & Christiaen, L. NK4 antagonizes Tbx1/10 to promote cardiac versus pharyngeal muscle fate in the ascidian second heart field. PLoS Biol. 11, e1001725 (2013). This paper identified an ontogenetic motif regulating cardiac and pharyngeal skeletal muscle development in C. intestinalis through asymmetric cell division events and anatagonistic interactions between conserved master regulators of cardiopharyngeal fate.
Razy-Krajka, F. et al. Collier/OLF/EBF-dependent transcriptional dynamics control pharyngeal muscle specification from primed cardiopharyngeal progenitors. Dev. Cell 29, 263–276 (2014). This paper demonstrated that the multipotent cardiopharyngeal progenitors of C. intestinalis are multilineage primed and activate both early heart and pharyngeal muscle regulators that segregate to their corresponding precursors following asymmetric cell divisions.
Harafuji, N., Keys, D. N. & Levine, M. Genome-wide identification of tissue-specific enhancers in the Ciona tadpole. Proc. Natl Acad. Sci. USA 99, 6802–6805 (2002).
Heude, E. et al. Jaw muscularization requires Dlx expression by cranial neural crest cells. Proc. Natl Acad. Sci. USA 107, 11441–11446 (2010).
Yasui, K., Kaji, T., Morov, A. R. & Yonemura, S. Development of oral and branchial muscles in lancelet larvae of Branchiostoma japonicum. J. Morphol. 275, 465–477 (2014).
Goldschmidt, R. Amphioxides. Wiss Ergeb Dtsch Tiefsee-Expedition [in German] 12, 1–92 (1905).
Holland, N. D., Venkatesh, T. V., Holland, L. Z., Jacobs, D. K. & Bodmer, R. AmphiNk2-tin, an amphioxus homeobox gene expressed in myocardial progenitors: insights into evolution of the vertebrate heart. Dev. Biol. 255, 128–137 (2003).
Mahadevan, N. R., Horton, A. C. & Gibson-Brown, J. J. Developmental expression of the amphioxus Tbx1/10 gene illuminates the evolution of vertebrate branchial arches and sclerotome. Dev. Genes Evol. 214, 559–566 (2004).
Jackman, W. R., Langeland, J. A. & Kimmel, C. B. islet reveals segmentation in the Amphioxus hindbrain homolog. Dev. Biol. 220, 16–26 (2000).
Belgacem, M. R., Escande, M. L., Escriva, H. & Bertrand, S. Amphioxus Tbx6/16 and Tbx20 embryonic expression patterns reveal ancestral functions in chordates. Gene Expr. Patterns 11, 239–243 (2011).
Willey, A. Amphioxus and the Ancestery of the Vertebrates (Macmillan, 1894).
Schubert, M., Meulemans, D., Bronner-Fraser, M., Holland, L. Z. & Holland, N. D. Differential mesodermal expression of two amphioxus MyoD family members (AmphiMRF1 and AmphiMRF2). Gene Expr. Patterns 3, 199–202 (2003).
Mazet, F., Masood, S., Luke, G. N., Holland, N. D. & Shimeld, S. M. Expression of AmphiCoe, an amphioxus COE/EBF gene, in the developing central nervous system and epidermal sensory neurons. Genesis 38, 58–65 (2004).
Holland, L. Z., Schubert, M., Kozmik, Z. & Holland, N. D. AmphiPax3/7, an amphioxus paired box gene: insights into chordate myogenesis, neurogenesis, and the possible evolutionary precursor of definitive vertebrate neural crest. Evol. Dev. 1, 153–165 (1999).
Hirano, T. & Nishida, H. Developmental fates of larval tissues after metamorphosis in ascidian Halocynthia roretzi. I. Origin of mesodermal tissues of the juvenile. Dev. Biol. 192, 199–210 (1997).
Tokuoka, M., Satoh, N. & Satou, Y. A bHLH transcription factor gene, Twist-like1, is essential for the formation of mesodermal tissues of Ciona juveniles. Dev. Biol. 288, 387–396 (2005).
Kuratani, S. Evolution of the vertebrate jaw from developmental perspectives. Evol. Dev. 14, 76–92 (2012).
Mallatt, J. The origin of the vertebrate jaw: neoclassical ideas versus newer, development-based ideas. Zoolog. Sci. 25, 990–998 (2008).
Valentine, J. W. On the Origin of Phyla (Univ. Chicago Press, 2004).
Gillis, J. A., Fritzenwanker, J. H. & Lowe, C. J. A stem-deuterostome origin of the vertebrate pharyngeal transcriptional network. Proc R. Soc. B 279, 237–246 (2012).
Haun, C., Alexander, J., Stainier, D.Y. & Okkema, P. G. Rescue of Caenorhabditis elegans pharyngeal development by a vertebrate heart specification gene. Proc. Natl Acad. Sci. USA. 95, 5072–5075 (1998).
Boukhatmi, H. et al. An Org-1-Tup transcriptional cascade reveals different types of alary muscles connecting internal organs in Drosophila. Development 141, 3761–3771 (2014).
Crozatier, M. & Vincent, A. Requirement for the Drosophila COE transcription factor Collier in formation of an embryonic muscle: transcriptional response to notch signalling. Development 126, 1495–1504 (1999).
Enriquez, J., de Taffin, M., Crozatier, M., Vincent, A. & Dubois, L. Combinatorial coding of Drosophila muscle shape by Collier and Nautilus. Dev. Biol. 363, 27–39 (2012).
Mann, T., Bodmer, R. & Pandur, P. The Drosophila homolog of vertebrate Islet1 is a key component in early cardiogenesis. Development 136, 317–326 (2009).
Schaub, C. & Frasch, M. Org-1 is required for the diversification of circular visceral muscle founder cells and normal midgut morphogenesis. Dev. Biol. 376, 245–259 (2013).
Schaub, C., Nagaso, H., Jin, H. & Frasch, M. Org-1, the Drosophila ortholog of Tbx1, is a direct activator of known identity genes during muscle specification. Development 139, 1001–1012 (2012).
Lescroart, F. & Meilhac, S. M. Cell lineages, growth and repair of the mouse heart. Results Probl. Cell Differ. 55, 263–289 (2012).
Lescroart, F., Mohun, T., Meilhac, S. M., Bennett, M. & Buckingham, M. Lineage tree for the venous pole of the heart: clonal analysis clarifies controversial genealogy based on genetic tracing. Circ. Res. 111, 1313–1322 (2012).
Lacalli, T. C. & Holland, L. Z. The developing dorsal ganglion of the salp Thalia democratica, and the nature of the ancestral chordate brain. Phil. Trans. R. Soc. Lond. B 353, 1943–1967 (1998).
Gee, H. in Major Events in Early Vertebrate Evolution: Palaeontology, Phylogeny, Genetics and Development (ed. Ahlberg, P. E.) 1–14 (Taylor & Francis, 2001).
Acknowledgements
We thank T. Miyashita and F. Razy-Krajka for their detailed reviews of the manuscript. We are thankful to the Dean of Howard University (HU) College of Medicine, M. Johnson, and the Chair of HU Department of Anatomy, D. Orlic, for helping to organize, financially and logistically, the First Evo-Devo Meeting On Heart and Head Muscles at HU (May, 2014) that led to the publication of this Review. We also thank the other participants at the workshop: A. Kahana, P. Okkema, A. Vincent, T. Hirasawa, S. Tajbakhsh, S. Dietrich and R. Knight. L.C. is supported by National Institutes of Health (NIH)/National Institute of General Medical Sciences (NIGMS) grant R01GM096032 and NIH/National Heart, Lung and Blood Instiute (NHLBI) grant R01HL108643, E.T. by the European Research Council and Israel Science Foundation, R.D. and J.Z. by HU College of Medicine, R.G.K. by Inserm, the Agence Nationale pour la Recherche, Association Française contre les Myopathies and Fondation pour la Recherche Médicale, and M.L. by NIH grant NS076542.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reprints and permissions information is available at www.nature.com/reprints.
Rights and permissions
About this article
Cite this article
Diogo, R., Kelly, R., Christiaen, L. et al. A new heart for a new head in vertebrate cardiopharyngeal evolution. Nature 520, 466–473 (2015). https://doi.org/10.1038/nature14435
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14435
This article is cited by
-
Sinus venosus adaptation models prolonged cardiovascular disease and reveals insights into evolutionary transitions of the vertebrate heart
Nature Communications (2023)
-
Fossil evidence for a pharyngeal origin of the vertebrate pectoral girdle
Nature (2023)
-
The second heart field: the first 20 years
Mammalian Genome (2023)
-
Single cell multi-omic analysis identifies a Tbx1-dependent multilineage primed population in murine cardiopharyngeal mesoderm
Nature Communications (2021)
-
Cardiopharyngeal deconstruction and ancestral tunicate sessility
Nature (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.