Abstract
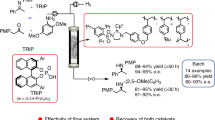
Chemical manufacturing is conducted using either batch systems or continuous-flow systems. Flow systems have several advantages over batch systems, particularly in terms of productivity, heat and mixing efficiency, safety, and reproducibility1,2,3,4. However, for over half a century, pharmaceutical manufacturing has used batch systems because the synthesis of complex molecules such as drugs has been difficult to achieve with continuous-flow systems5,6. Here we describe the continuous-flow synthesis of drugs using only columns packed with heterogeneous catalysts. Commercially available starting materials were successively passed through four columns containing achiral and chiral heterogeneous catalysts to produce (R)-rolipram7, an anti-inflammatory drug and one of the family of γ-aminobutyric acid (GABA) derivatives8. In addition, simply by replacing a column packed with a chiral heterogeneous catalyst with another column packed with the opposing enantiomer, we obtained antipole (S)-rolipram. Similarly, we also synthesized (R)-phenibut, another drug belonging to the GABA family. These flow systems are simple and stable with no leaching of metal catalysts. Our results demonstrate that multistep (eight steps in this case) chemical transformations for drug synthesis can proceed smoothly under flow conditions using only heterogeneous catalysts, without the isolation of any intermediates and without the separation of any catalysts, co-products, by-products, and excess reagents. We anticipate that such syntheses will be useful in pharmaceutical manufacturing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wiles, C. & Watts, P. Continuous flow reactors: a perspective. Green Chem. 14, 38–54 (2012)
Ley, S. V. & Baxendale, I. R. New tools and concepts for modern organic synthesis. Nature Rev. Drug Discov. 1, 573–586 (2002)
Geyer, K., Codée, J. D. C. & Seeberger, P. H. Microreactors as tools for synthetic chemists—the chemists’ round-bottomed flask of the 21st century? Chemistry 12, 8434–8442 (2006)
Hartman, R. L., McMullen, J. P. & Jensen, K. F. Deciding whether to go with the flow: evaluating the merits of flow reactors for synthesis. Angew. Chem. Int. Ed. 50, 7502–7519 (2011)
Van Arnum, P. Advancing flow chemistry in API manufacturing. Pharm. Technol. 37, 78–82 (2013)
Poechlauer, P. et al. Continuous processing in the manufacture of active pharmaceutical ingredients and finished dosage forms: an industry perspective. Org. Process Res. Dev. 16, 1586–1590 (2012)
Sommer, N. et al. The antidepressant rolipram suppresses cytokine production and prevents autoimmune encephalomyelitis. Nature Med. 1, 244–248 (1995)
Macdonald, R. L. & Olsen, R. W. GABAA receptor channels. Annu. Rev. Neurosci. 17, 569–602 (1994)
Anastas P. T., Warner J. C., eds. Green Chemistry Theory and Practice (Oxford Univ. Press, 1998)
Kirschning, A., Solodenko, W. & Mennecke, K. Combining enabling techniques in organic synthesis: continuous flow processes with heterogenized catalysts. Chemistry 12, 5972–5990 (2006)
Frost, C. G. & Mutton, L. Heterogeneous catalytic synthesis using microreactor technology. Green Chem. 12, 1687–1703 (2010)
Tsubogo, T., Ishiwata, T. & Kobayashi, S. Asymmetric carbon–carbon bond formation under continuous-flow conditions with chiral heterogeneous catalysts. Angew. Chem. Int. Ed. 52, 6590–6604 (2013)
Battilocchio, C., Hawkins, J. M. & Ley, S. V. A mild and efficient flow procedure for the transfer hydrogenation of ketones and aldehydes using hydrous zirconia. Org. Lett. 15, 2278–2281 (2013)
Pastre, J. C., Browne, D. L. & Ley, S. V. Flow chemistry syntheses of natural products. Chem. Soc. Rev. 42, 8849–8869 (2013)
Hartwig, J. et al. Heating under high-frequency inductive conditions: application to the continuous synthesis of the neurolepticum olanzapine (Zyprexa). Angew. Chem. Int. Ed. 52, 9813–9817 (2013)
Webb, D. & Jamison, T. F. Continuous flow multi-step organic synthesis. Chem. Sci. 1, 675–680 (2010)
Hopkin, M. D., Baxendale, I. R. & Ley, S. V. An expeditious synthesis of imatinib and analogues utilising flow chemistry methods. Org. Biomol. Chem. 11, 1822–1839 (2013)
Barnes, D. M. et al. Development of a catalytic enantioselective conjugate addition of 1,3-dicarbonyl compounds to nitroalkenes for the synthesis of endothelin-A antagonist ABT-546. Scope, mechanism, and further application to the synthesis of the antidepressant rolipram. J. Am. Chem. Soc. 124, 13097–13105 (2002)
Day, J. P. et al. Elucidation of a structural basis for the inhibitor-driven, p62 (SQSTM1)-dependent intracellular redistribution of cAMP phosphodiesterase-4A4 (PDE4A4). J. Med. Chem. 54, 3331–3347 (2011)
Maxwell, C. R., Kanes, S. J., Abel, T. & Siegel, S. J. Phosphodiesterase inhibitors: a novel mechanism for receptor-independent antipsychotic medications. Neuroscience 129, 101–107 (2004)
Malykh, A. G. & Sadaie, M. R. Piracentam and piracetam-like drugs. Drugs 70, 287–312 (2010)
Motokura, K., Tada, M. & Iwasawa, Y. Layered materials with coexisting acidic and basic sites for catalytic one-pot reaction sequences. J. Am. Chem. Soc. 131, 7944–7945 (2009)
Soldi, L. et al. Use of immobilized organic base catalysts for continuous-flow fine chemical synthesis. J. Catal. 258, 289–295 (2008)
Worrall, D. E. Nitrostyrene. Org. Synth. 9, 66–69 (1929)
Ojima I., ed. Catalytic Asymmetric Synthesis 3rd edn (Wiley, 2010)
Tsubogo, T., Yamashita, Y. & Kobayashi, S. Toward efficient asymmetric carbon–carbon bond formation: continuous flow with chiral heterogeneous catalysts. Chemistry 18, 13624–13628 (2012)
Kobayashi, J. et al. A microfluidic device for conducting gas–liquid–solid hydrogenation reactions. Science 304, 1305–1308 (2004)
O’Brien, M. et al. Hydrogenation in flow: homogeneous and heterogeneous catalysis using Teflon AF-2400 to effect gas-liquid contact at elevated pressure. Chem. Sci. 2, 1250–1257 (2011)
Hynes, P. S., Stupple, P. A. & Dixon, D. J. Organocatalytic asymmetric total synthesis of (R)-rolipram and formal synthesis of (3S,4R)-paroxetine. Org. Lett. 10, 1389–1391 (2008)
Oyamada, H., Naito, T. & Kobayashi, S. Continuous flow hydrogenation using polysilane-supported palladium/alumina hybrid catalysts. Beilstein J. Org. Chem. 7, 735–739 (2011)
Acknowledgements
This work was partially supported by a Grant-in-Aid for Science Research from the Japan Society for the Promotion of Science (JSPS), Global COE Program, The University of Tokyo, MEXT, Japan, the ACT-C and Center of Innovation (COI) Program, and the Japan Science and Technology Agency (JST).
Author information
Authors and Affiliations
Contributions
T.T. and H.O. designed and performed the experiments. S.K. conceived, designed and directed the investigations and wrote the manuscript with revisions provided by T.T.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text, Methods and Data, Supplementary Figures 1-5, and additional references. (PDF 1053 kb)
Rights and permissions
About this article
Cite this article
Tsubogo, T., Oyamada, H. & Kobayashi, S. Multistep continuous-flow synthesis of (R)- and (S)-rolipram using heterogeneous catalysts. Nature 520, 329–332 (2015). https://doi.org/10.1038/nature14343
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14343
This article is cited by
-
Practical synthesis of tetrahydrofolate by highly efficient catalytic hydrogenation in continuous flow
Journal of Flow Chemistry (2024)
-
Ex-situ generation and synthetic utilization of bare trifluoromethyl anion in flow via rapid biphasic mixing
Nature Communications (2023)
-
Impact of gas-solid direct contact on gas-liquid-solid reaction performance in a flow reactor
Journal of Flow Chemistry (2023)
-
Geminal-atom catalysis for cross-coupling
Nature (2023)
-
Direct C–H metallation of tetrahydrofuran and application in flow
Nature Synthesis (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.