Abstract
Cancer cells adapt their metabolic processes to support rapid proliferation, but less is known about how cancer cells alter metabolism to promote cell survival in a poorly vascularized tumour microenvironment1,2,3. Here we identify a key role for serine and glycine metabolism in the survival of brain cancer cells within the ischaemic zones of gliomas. In human glioblastoma multiforme, mitochondrial serine hydroxymethyltransferase (SHMT2) and glycine decarboxylase (GLDC) are highly expressed in the pseudopalisading cells that surround necrotic foci. We find that SHMT2 activity limits that of pyruvate kinase (PKM2) and reduces oxygen consumption, eliciting a metabolic state that confers a profound survival advantage to cells in poorly vascularized tumour regions. GLDC inhibition impairs cells with high SHMT2 levels as the excess glycine not metabolized by GLDC can be converted to the toxic molecules aminoacetone and methylglyoxal. Thus, SHMT2 is required for cancer cells to adapt to the tumour environment, but also renders these cells sensitive to glycine cleavage system inhibition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
15 April 2015
A minor change was made to the legend of Extended Data Fig. 5 to clarify the publisher on a figure credit.
References
Cantor, J. R. & Sabatini, D. M. Cancer cell metabolism: one hallmark, many faces. Cancer Discov 2, 881–898 (2012)
Tennant, D. A., Duran, R. V. & Gottlieb, E. Targeting metabolic transformation for cancer therapy. Nature Rev. Cancer 10, 267–277 (2010)
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009)
Mattson, M. P. & Shea, T. B. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 26, 137–146 (2003)
Saudubray, J. M., Van den Berghe, G. & Walter, J. Inborn metabolic diseases: diagnosis and treatment 5th edn (Springer, 2012)
Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 (2001)
Zhang, W. C. et al. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell 148, 259–272 (2012)
Tibbetts, A. S. & Appling, D. R. Compartmentalization of Mammalian folate-mediated one-carbon metabolism. Annu. Rev. Nutr. 30, 57–81 (2010)
Chen, J., McKay, R. M. & Parada, L. F. Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell 149, 36–47 (2012)
Lee, J. et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 9, 391–403 (2006)
Shikano, N. et al. Stimulation of 125I-3-iodo-α-methyl-l-tyrosine uptake in Chinese hamster ovary (CHO-K1) cells by tyrosine esters. Nucl. Med. Biol. 37, 189–196 (2010)
Dale, R. A. Catabolism of threonine in mammals by coupling of l-threonine 3-dehydrogenase with 2-amino-3-oxobutyrate-CoA ligase. Biochim. Biophys. Acta 544, 496–503 (1978)
Tressel, T., Thompson, R., Zieske, L. R., Menendez, M. I. & Davis, L. Interaction between l-threonine dehydrogenase and aminoacetone synthetase and mechanism of aminoacetone production. J. Biol. Chem. 261, 16428–16437 (1986)
Sartori, A. et al. Aminoacetone, a putative endogenous source of methylglyoxal, causes oxidative stress and death to insulin-producing RINm5f cells. Chem. Res. Toxicol. 21, 1841–1850 (2008)
Kalapos, M. P. Methylglyoxal in living organisms: chemistry, biochemistry, toxicology and biological implications. Toxicol. Lett. 110, 145–175 (1999)
Labuschagne, C. F., van den Broek, N. J., Mackay, G. M., Vousden, K. H. & Maddocks, O. D. Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Rep. 7, 1248–1258 (2014)
Jain, M. et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science 336, 1040–1044 (2012)
Narkewicz, M. R., Sauls, S. D., Tjoa, S. S., Teng, C. & Fennessey, P. V. Evidence for intracellular partitioning of serine and glycine metabolism in Chinese hamster ovary cells. Biochem. J. 313, 991–996 (1996)
Rong, Y., Durden, D. L., Van Meir, E. G. & Brat, D. J. ‘Pseudopalisading’ necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J. Neuropathol. Exp. Neurol. 65, 529–539 (2006)
Nelson, D. A. et al. Hypoxia and defective apoptosis drive genomic instability and tumorigenesis. Genes Dev. 18, 2095–2107 (2004)
Chaneton, B. et al. Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature 491, 458–462 (2012)
Gui, D. Y., Lewis, C. A. & Vander Heiden, M. G. Allosteric regulation of PKM2 allows cellular adaptation to different physiological states. Sci. Signal. 6, pe7 (2013)
Keller, K. E., Tan, I. S. & Lee, Y. S. SAICAR stimulates pyruvate kinase isoform M2 and promotes cancer cell survival in glucose-limited conditions. Science 338, 1069–1072 (2012)
Anastasiou, D. et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334, 1278–1283 (2011)
Christofk, H. R. et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452, 230–233 (2008)
Anastasiou, D. et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nature Chem. Biol. 8, 839–847 (2012)
Lunt, S. Y. & Vander Heiden, M. G. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 27, 441–464 (2011)
Papandreou, I., Cairns, R. A., Fontana, L., Lim, A. L. & Denko, N. C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 3, 187–197 (2006)
Brown, J. M. & Wilson, W. R. Exploiting tumour hypoxia in cancer treatment. Nature Rev. Cancer 4, 437–447 (2004)
Mehta, S. et al. The central nervous system-restricted transcription factor Olig2 opposes p53 responses to genotoxic damage in neural progenitors and malignant glioma. Cancer Cell 19, 359–371 (2011)
Chudnovsky, Y. et al. ZFHX4 interacts with the NuRD core member CHD4 and regulates the glioblastoma tumor-initiating cell state. Cell Rep 6, 313–324 (2014)
Possemato, R. et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476, 346–350 (2011)
Rhodes, D. R. et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia 6, 1–6 (2004)
Luo, B. et al. Highly parallel identification of essential genes in cancer cells. Proc. Natl Acad. Sci. USA 105, 20380–20385 (2008)
Shalem, O. et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87 (2014)
Wang, T., Wei, J. J., Sabatini, D. M. & Lander, E. S. Genetic screens in human cells using the CRISPR-Cas9 system. Science 343, 80–84 (2014)
Ptolemy, A. S. et al. A 9-month-old boy with seizures and discrepant urine tryptophan concentrations. Clin. Chem. 57, 545–548 (2011)
Kami, K. et al. Metabolomic profiling of lung and prostate tumor tissues by capillary electrophoresis time-of-flight mass spectrometry. Metabolomics 9, 444–453 (2013)
Xiao, S. & Yu, P. H. A fluorometric high-performance liquid chromatography procedure for simultaneous determination of methylamine and aminoacetone in blood and tissues. Anal. Biochem. 384, 20–26 (2009)
Kazachkov, M. & Yu, P. H. A novel HPLC procedure for detection and quantification of aminoacetone, a precursor of methylglyoxal, in biological samples. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 824, 116–122 (2005)
Rotem, R. et al. Jasmonates: novel anticancer agents acting directly and selectively on human cancer cell mitochondria. Cancer Res. 65, 1984–1993 (2005)
Jois, M., Hall, B., Fewer, K. & Brosnan, J. T. Regulation of hepatic glycine catabolism by glucagon. J. Biol. Chem. 264, 3347–3351 (1989)
Brat, D. J. & Van Meir, E. G. Vaso-occlusive and prothrombotic mechanisms associated with tumor hypoxia, necrosis, and accelerated growth in glioblastoma. Lab. Invest. 84, 397–405 (2004).
Acknowledgements
We thank members of the Sabatini laboratory for assistance and feedback, in particular Y. Shaul, T. Wang, S. Wang and O. Yilmaz. Authors would like to thank J. Taylor for GBM sample collection, and T. DiCesare for illustrations. This work was supported by a Basic Research Fellowship from the American Brain Tumor Association to D.K.; MIT School of Science Fellowship in Cancer Research and National Institutes of Health (NIH) T32GM007287 to B.P.F., fellowships from the Jane Coffin Childs Memorial Fund and Leukemia and Lymphoma Society to K.B.; a grant from the NIH (K99 CA168940) to R.P.; an American Cancer Society fellowship and an American Brain Tumor Association Discovery Grant to Y.C.; a fellowship from the US National Institute of Aging to W.W.C.; NIH (K08-NS087118) to S.H.R.; support from NIH (R01CA168653, 5P30CA14051), the Smith Family Foundation, the Burroughs Wellcome Fund, the Damon Runyon Cancer Research Foundation, and the Stern family to M.G.V.H.; DOD CDMRP Discovery Award, grants from the David H. Koch Institute for Integrative Cancer Research at MIT, The Alexander and Margaret Stewart Trust Fund, and NIH (CA103866, CA129105, and AI07389) to D.M.S.; D.M.S. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
D.K. and D.M.S. conceived the study and designed most of the experiments. D.K. performed most of the experiments (cell viability and proliferation, western blotting, immunohistochemistry, xenografts) with assistance from K.B., R.L.P., Y.C., W.W.C., S.K. and M.K.; B.P.F. and M.G.V.H. designed, carried out and analysed pyruvate kinase activity and LC–MS based experiments with input and assistance from E.F., M.E.P. and D.Y.G.; E.F., D.K. and J.R.C. designed and carried out LC–MS-based derivatization experiments measuring aminoacetone levels. K.K. and L.M.S. conducted and analysed CE–MS metabolite profiling. M.S. provided GBM sections and conducted analyses and imaging of IHC. S.H.R. and K.L.L. assisted with neurosphere-forming cell characterizations. D.K. and D.M.S. wrote and all authors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
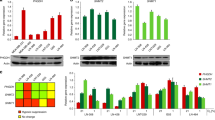
Extended Data Figure 1 GLDC and SHMT2 expression and function in neurosphere-forming cells.
a, Micrographs of cells (0308 cell line) cultured under neurosphere-forming conditions (top panel) or differentiated into their non-tumorigenic counterparts (bottom panel). b, Immunoblots from neurosphere-forming cells maintained in the neurosphere state or differentiated by serum treatment for 1 week, with SOX-2 as a marker for the neural stem cell-like state. c, Immunoblots from neurosphere-forming cells transduced with control shRNA (shGFP), or NOTCH2 shRNAs, which induce differentiation, for 1 week. d, Immunoblot showing suppression of GLDC expression in BT145 cells transduced with the indicated shRNAs for 5 days. e, Micrographs from undifferentiated or serum-differentiated BT145 cells expressing shGFP or GLDC shRNAs for 6 days. f, Viability of LN229 cells overexpressing blank vector or mouse GLDC, either untreated or treated with 1 mM esterified glycine for 3.5 days as indicated. Values are relative to that of the same cells left untreated. g, Immunoblot showing suppression of SHMT2 expression in BT145 cells transduced with the indicated shRNAs for 5 days. h, Immunoblots for SHMT2 expression in cells maintained in the neurosphere-forming state or induced to differentiate by serum treatment, which are from the same blot as shown in Fig. 1b. i, Immunoblots for SHMT2 expression in neurosphere-forming cells transduced with control (shGFP) or NOTCH2 shRNAs, which induce differentiation, for 1 week, and are from the same blot as shown in Fig. 1c. j, Micrographs showing morphology of BT145 cells transduced with the indicated shRNAs for 6 days. k, Cell viability of 0308 cells transduced with the indicated shRNAs for 6 days. Values are normalized to the viability of shGFP transduced cells. l, Clonogenic sphere formation in 0308 cells transduced with the indicated shRNAs and then plated as single cells. The proportion of wells containing single cells that were able to form spheres are shown as values relative to shGFP-transduced cells. For l, n = 2 independent biological replicates. For f and k, n = 3 independent biological replicates; error bars are s.d. *P < 0.05 (Student’s t-test).
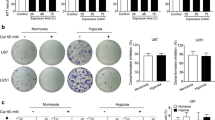
Extended Data Figure 2 Identification of GCAT as a mediator of toxicity caused by GLDC suppression.
a, Schematic presentation of pooled shRNA screen carried out in LN229 cells. Detailed procedures are provided in Methods. b, Table of a subset of genes examined in the pooled screen, the average fold change increase in relative abundance of all shRNAs for each gene in GLDC-suppressed conditions (shGLDC_1 and shGLDC_2) compared to the set of nontargeting control shRNAs, as described in Supplementary methods. Genes are sorted by ascending P value; the top 15 out of the 25 genes are shown. Asterisks indicate metabolic genes which do not function in glycine metabolism, included as additional controls. All shRNAs used and their abundance in each condition are shown in Supplementary Table 4. c, Relative representation, in the shGLDC infected pool, of each shRNA against non-targeting controls, SHMT2, and GCAT. A value of 1.0 indicates the average for all hairpins in the screen. Representation in both shGLDC_1 and shGLDC_2 are shown, so each hairpin is represented twice in the plot. Bars are mean ± s.e.m. *P < 0.05 (Student’s t-test). d, Immunoblots of LN229 cells transduced with shRNAs against GCAT as indicated. e, Extracted ion chromatogram showing peaks from FMOC derivatized aminoacetone and ethylamine (an internal standard spiked into each sample as a control for efficiency of derivatization, and recovery and detection) from pure standards (lower graph) and from a representative LN229 xenograft tumour sample (upper graph), showing a match between predicted and observed m/z values and retention times. f, Aminoacetone levels in control (no shRNA) cells, cells transduced with shGLDCdox, and cells with shGLDCdox plus shRNA-resistant mouse GLDC, which were all induced with doxycycline for 5 days; n = 3 independent biological replicates; error bars are s.d. *P < 0.05 (Student’s t-test). g, Methylglyoxal levels (argpyrimidine antibody) in LN229 cells transduced with Cas9 and single guide RNAs against GLDC for 7 days. h, Expression of GCAT in cells transduced with Cas9 and single guide RNAs against GCAT for 7 days. i, Methylglyoxal levels (argpyrimidine antibody) in LN229 cells transduced with Cas9 and the indicated single guide RNAs for 7 days, then secondarily transduced with shGLDC_2 for 5 days.
Extended Data Figure 3 Effects of glycine cleavage system inhibition on cells with high or low SHMT2 expression levels.
a, Overview of the serine hydroxymethyltransferase and glycine cleavage reactions mediated by SHMT2 and GLDC, respectively. Asterisks indicate metabolites that are labelled with 14C during mitochondrial metabolism of U-[14C]serine. Only upon completion of both reactions will 14C-labelled CO2 be formed, which is captured and detected by scintillation as described in Methods. b, Measurement of 14CO2 production, a readout of sequential SHMT2 and GLDC activity on U-[14C]serine, in intact mitochondria isolated from LN229 s expressing shGFP or shSHMT2_1, as described in Methods. c, Table indicating cell viability in various cell lines following transduction of GLDC shRNAs for 6–7 days . Values are relative to the CTG signal of the same cells secondarily transduced with shGFP and grown in parallel. d, SHMT2 and GLDC expression in LN229 cells stably transduced with shSHMT2_1 or shGFP, then secondarily transduced shGLDC or shGFP as indicated. e, Viability of LN229 cells first transduced with control or SHMT2 shRNAs, then transduced with shGFP or GLDC shRNAs for 5 days. Values are relative to that of cells secondarily transduced with shGFP. f, Viability of U251 cells first transduced with control or SHMT2 shRNAs, then transduced with shGFP or GLDC shRNAs for 7 days. Values are relative to that of cells secondarily transduced with shGFP. g–i, Viability of various cell lines transduced with shRNAs targeting (g) GLDC, (h) GCSH (glycine cleavage system protein H, another integral component of the glycine cleavage system), or (i) SHMT2. Values are relative to those of the cells expressing shGFP, which were grown in parallel. For b, c, and e–i, n = 3 independent biological replicates; error bars are s.d. *P < 0.05 (Student’s t-test).
Extended Data Figure 4 GLDC and SHMT2 expression in GBM tumours.
a, SHMT2 and GLDC expression across GBM and normal brain regions as examined in autopsy sections. A whole coronal section is shown, with the GBM bulk tumour outlined in white. Insets indicate magnified micrographs from the regions, indicated by the small red squares, from the same brain. For the bulk tumour insets, cells around the pseudopalisading necroses are shown. Scale bars for whole coronal section = 1 cm, and for insets = 100 µm. b, SHMT2 expression at the GBM-normal brain interface, showing SHMT2-immunoreactivity in migrating cells. Scale bar, 100 μm. c, Magnified image of the boxed region in b. d, e, High-magnification micrographs of SHMT2 and GLDC expression, respectively, in (1) perinecrotic GBM tumour, (2) non-ischaemic bulk tumour, (3) frontal cortex, (4) temporal white matter, and (5) striatum from autopsy cases. Scale bar, 100 μm. f, Semi-quantitative scoring of GLDC staining intensity by neuropathologist (M.S.) on 7 tumour biopsy cases (left) and 7 autopsy cases (right). g, Semi-quantitative scoring of SHMT2 staining intensity by neuropathologist (M.S.) on 7 tumour biopsy cases (left) and 7 autopsy cases (right). h, Viability of LN229 cells expressing an empty vector control or mouse GLDC cDNA and cultured in 0.5% hypoxia for 8 days. Values are relative to that of the same cells cultured in parallel in normoxia. i, Cell number counts from LN229 cells expressing an empty vector control or SHMT2 cDNA and cultured in 0.5% hypoxia for 8 days. Values are relative to the counts of the same cells cultured in parallel in normoxia. j, Viability of U251 cells expressing shRNA or cDNAs as indicated and cultured in 0.5% hypoxia for 6 days. Values are relative to that of the same cells cultured in parallel in normoxia. For f and g, bars indicate the mean. For h–j, n = 3 independent biological replicates; error bars are s.d. *P < 0.05 (Student’s t-test).
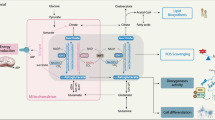
Extended Data Figure 5 Effects of SHMT2 expression on PKM2 activity and cell metabolism.
a, Absolute quantitative CE–MS measurement of intracellular metabolites from LN229 cells stably expressing indicated shRNAs without media change for 84 h. The metabolites with the greatest fold change are listed, in units of pmol per 106 cells. All metabolites listed are changed in a statistically significant manner (Student’s t-test, P < 0.05). Data for all 116 metabolites in the analysis are in Supplementary Table 5. b, Oxygen consumption in LN229 cells (in RPMI media) transduced with indicated shRNAs and cDNAs. Error bars are s.d. (n = 4 technical replicates). c, Pyruvate kinase activity assay from lysates of U251 cells transduced with indicated shRNAs or cDNAs. d, Proposed mechanism by which SHMT2 inhibition could lead to an increase in AICAR and SAICAR. In cells with high SHMT2 expression, 10-formyltetrahydrofolate, which is a downstream product of SHMT2 activity, serves as a cofactor for cytosolic 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase (ATIC) in the conversion of AICAR to FAICAR during purine biosynthesis. In the absence of SHMT2, a lack 10-formyltetrahydrofolate production could lead to accumulation of AICAR and SAICAR. As indicated by the asterisk, the contribution of SHMT2 to cytosolic 10-formyltetrahydrofolate formation may be direct, occur via export of formate from the mitochondria, or occur indirectly by changing serine/glycine levels and thereby altering SHMT1 activity. e, 13C labelling rates of pyruvate and its downstream metabolites in cells from h. Labelling rates are relative and expressed as fold changes in cells transduced with shSHMT2_1 versus cells transduced with shGFP, with or without RNAi-resistant SHMT2 cDNA. Estimated PK flux is the calculated total flux to the four product species in moles of 13C per unit time. Plots for individual labelled species over time are shown in g and Supplementary Table 9. f, Scheme for calculating total PK flux by measuring the net molar labelling of U-[13C]glucose derived, PK product species. Estimated PK flux is the calculated total flux to the four product species in moles of 13C per unit time. g, Plots of glucose-derived labelled species abundance over time for shGFP, shSHMT2, and shGFP plus PKM2 cDNA expressing LN229 cells shown in Fig. 4e–g. Raw data, calculations and plots for all stable cell lines are shown in Supplementary data Tables 8 and 9. h, SHMT2 and PKM2 expression in LN229 cells transduced with shRNAs and cDNAs as indicated. Asterisk indicates the overexpressed PKM2, which shows higher migration due to Flag tag. i, Oxygen consumption in LN229 cells expressing shGFP or shSHMT2_1 in RPMI media with or without 1 mM pyruvate. Error bars are s.d. (n = 4 technical replicates). j, Viability of LN229 cells transduced with shRNAs or cDNAs as indicated, and also treated with vehicle or 50 μM of TEPP-46 or DASA-58 as indicated, then subjected to hypoxia for 6 days. Values are relative to the same cells grown in parallel in normoxia. k, Overview of effects of SHMT2 expression on cell metabolism, tumour cell survival, and liability to toxic glycine accumulation. Red arrow indicates upregulation, and blue arrow indicates downregulation. Grey bar indicates the inhibitory effect of SHMT2 activity on PKM2 activity. Depiction of pseudopalisading necrosis is adapted from ref.43, Nature Publishing Group. Illustration by Mica Duran. For a, c, e, g, and j, n = 3 independent biological replicates; error bars are s.d. *P < 0.05 (Student’s t-test).
Supplementary information
Supplementary Information
This file contains a Supplementary Figure showing uncropped blots with size marker indications. (PDF 472 kb)
Supplementary Table 1
A list of genes whose loss causes toxicity in the developing brain, and the associated disorder, targeted organs, and other relevant information. (XLSX 14 kb)
Supplementary Table 2
This table contains data from oncomine-based gene expression studies comparing glioma vs. normal brain, and list of metabolic genes that are highly overexpressed in gliomas in each study. (XLSX 152 kb)
Supplementary Table 3
This table contains a data summary for gene expression omnibus microarray-based studies comparing expression of select genes in neural stem cells versus differentiated controls. (XLSX 13 kb)
Supplementary Table 4
A list of individual shRNAs used and abundance scores for each shRNA under each pool condition. (XLSX 37 kb)
Supplementary Table 5
This table contains Data for all metabolites in LN229 cells expressing shGFP or shSHMT2_1 as measured using CE-MS based, quantitative metabolite profiling. (XLSX 38 kb)
Supplementary Table 6
A list of metabolites identified through LC-MS based, untargeted discovery using Progenesis software, in positive mode. (XLSX 1194 kb)
Supplementary Table 7
A list of metabolites identified through LC-MS based, untargeted discovery using Progenesis software, in negative mode. (XLSX 606 kb)
Supplementary Table 8
This table contains methods and data for 13-C labelled species in the labelling rate analyses shown in Figure 4e-g and Extended Figure 5c-d. (XLSX 288 kb)
Supplementary Table 9
This table contains plots for 13-C labelled species abundance over time for the labelling rate analyses shown in Figure 4e-g and Extended Figure 5c-d. (XLSX 58 kb)
Rights and permissions
About this article
Cite this article
Kim, D., Fiske, B., Birsoy, K. et al. SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature 520, 363–367 (2015). https://doi.org/10.1038/nature14363
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14363
This article is cited by
-
Hypoxia-induced SHMT2 protein lactylation facilitates glycolysis and stemness of esophageal cancer cells
Molecular and Cellular Biochemistry (2024)
-
Unraveling the role of the mitochondrial one-carbon pathway in undifferentiated thyroid cancer by multi-omics analyses
Nature Communications (2024)
-
SHMT2 regulates esophageal cancer cell progression and immune Escape by mediating m6A modification of c-myc
Cell & Bioscience (2023)
-
Impairments in SHMT2 expression or cellular folate availability reduce oxidative phosphorylation and pyruvate kinase activity
Genes & Nutrition (2023)
-
Disruption of sugar nucleotide clearance is a therapeutic vulnerability of cancer cells
Nature (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.