Abstract
A defining feature of vertebrates (craniates) is a pronounced head that is supported and protected by a robust cellular endoskeleton. In the first vertebrates, this skeleton probably consisted of collagenous cellular cartilage, which forms the embryonic skeleton of all vertebrates and the adult skeleton of modern jawless and cartilaginous fish. In the head, most cellular cartilage is derived from a migratory cell population called the neural crest, which arises from the edges of the central nervous system. Because collagenous cellular cartilage and neural crest cells have not been described in invertebrates1, the appearance of cellular cartilage derived from neural crest cells is considered a turning point in vertebrate evolution2. Here we show that a tissue with many of the defining features of vertebrate cellular cartilage transiently forms in the larvae of the invertebrate chordate Branchiostoma floridae (Florida amphioxus). We also present evidence that during evolution, a key regulator of vertebrate cartilage development, SoxE, gained new cis-regulatory sequences that subsequently directed its novel expression in neural crest cells. Together, these results suggest that the origin of the vertebrate head skeleton did not depend on the evolution of a new skeletal tissue, as is commonly thought, but on the spread of this tissue throughout the head. We further propose that the evolution of cis-regulatory elements near an ancient regulator of cartilage differentiation was a major factor in the evolution of the vertebrate head skeleton.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Medeiros, D. M. The evolution of the neural crest: new perspectives from lamprey and invertebrate neural crest-like cells. Wiley Interdiscip. Rev. Dev. Biol. 2, 1–15 (2013)
Gans, C. & Northcutt, R. G. Neural crest and the origin of vertebrates: a new head. Science 220, 268–273 (1983)
Witten, P. E., Huysseune, A. & Hall, B. K. A practical approach for the identification of the many cartilaginous tissues in teleost fish. J. Appl. Ichthyology 26, 257–262 (2010)
Cole, A. G. & Hall, B. K. The nature and significance of invertebrate cartilages revisited: distribution and histology of cartilage and cartilage-like tissues within the Metazoa. Zoology (Jena) 107, 261–273 (2004)
Rychel, A. L. & Swalla, B. J. Development and evolution of chordate cartilage. J. Exp. Zool. 308B, 325–335 (2007)
Wright, G. M., Keeley, F. W. & Robson, P. The unusual cartilaginous tissues of jawless craniates, cephalochordates and invertebrates. Cell Tissue Res. 304, 165–174 (2001)
Kaneto, S. & Wada, H. Regeneration of amphioxus oral cirri and its skeletal rods: implications for the origin of the vertebrate skeleton. J. Exp. Zool. 316B, 409–417 (2011)
Paris, M. et al. Amphioxus postembryonic development reveals the homology of chordate metamorphosis. Curr. Biol. 18, 825–830 (2008)
Walshe, J. & Mason, I. Fgf signalling is required for formation of cartilage in the head. Dev. Biol. 264, 522–536 (2003)
Jandzik, D. et al. Roles for FGF in lamprey pharyngeal pouch formation and skeletogenesis highlight ancestral functions in the vertebrate head. Development 141, 629–638 (2014)
Mohammadi, M. et al. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science 276, 955–960 (1997)
Favata, M. F. et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273, 18623–18632 (1998)
Bi, W. M., Deng, J. M., Zhang, Z. P., Behringer, R. R. & de Crombrugghe, B. Sox9 is required for cartilage formation. Nature Genet. 22, 85–89 (1999)
Lefebvre, V., Li, P. & de Crombrugghe, B. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 17, 5718–5733 (1998)
Kumar, M., Ray, P. & Chapman, S. C. Fibroblast growth factor and bone morphogenetic protein signaling are required for specifying prechondrogenic identity in neural crest-derived mesenchyme and initiating the chondrogenic program. Dev. Dyn. 241, 1091–1103 (2012)
McCauley, D. W. & Bronner-Fraser, M. Importance of SoxE in neural crest development and the evolution of the pharynx. Nature 441, 750–752 (2006)
Ohtani, K. et al. Expression of Sox and fibrillar collagen genes in lamprey larval chondrogenesis with implications for the evolution of vertebrate cartilage. J. Exp. Zool. 310B, 596–607 (2008)
Znosko, W. A. et al. Overlapping functions of Pea3 ETS transcription factors in FGF signaling during zebrafish development. Dev. Biol. 342, 11–25 (2010)
Li, C. Y., Scott, D. A., Hatch, E., Tian, X. Y. & Mansour, S. L. Dusp6 (Mkp3) is a negative feedback regulator of FGF-stimulated ERK signaling during mouse development. Development 134, 167–176 (2007)
Conklin, E. G. The embryology of amphioxus. J. Morphol. 54, 69–151 (1932)
Betancur, P., Bronner-Fraser, M. & Sauka-Spengler, T. Genomic code for Sox10 activation reveals a key regulatory enhancer for cranial neural crest. Proc. Natl Acad. Sci. USA 107, 3570–3575 (2010)
Van Otterloo, E. et al. Novel Tfap2-mediated control of soxE expression facilitated the evolutionary emergence of the neural crest. Development 139, 720–730 (2012)
Lakiza, O., Miller, S., Bunce, A., Lee, E. M. J. & McCauley, D. W. SoxE gene duplication and development of the lamprey branchial skeleton: insights into development and evolution of the neural crest. Dev. Biol. 359, 149–161 (2011)
Sauka-Spengler, T., Meulemans, D., Jones, M. & Bronner-Fraser, M. Ancient evolutionary origin of the neural crest gene regulatory network. Dev. Cell 13, 405–420 (2007)
Cossais, F. et al. Replacement of mouse Sox10 by the Drosophila ortholog Sox100B provides evidence for co-option of SoxE proteins into vertebrate-specific gene-regulatory networks through altered expression. Dev. Biol. 341, 267–281 (2010)
Manzanares, M. et al. Conservation and elaboration of Hox gene regulation during evolution of the vertebrate head. Nature 408, 854–857 (2000)
Meulemans, D. & Bronner-Fraser, M. Insights from amphioxus into the evolution of vertebrate cartilage. PLoS ONE 2, e787 (2007)
Li, W. & Cornell, R. A. Redundant activities of Tfap2a and Tfap2c are required for neural crest induction and development of other non-neural ectoderm derivatives in zebrafish embryos. Dev. Biol. 304, 338–354 (2007)
Chen, J. Y., Huang, D. Y. & Li, C. W. An early Cambrian craniate-like chordate. Nature 402, 518–522 (1999)
Paris, M. et al. Amphioxus postembryonic development reveals the homology of chordate metamorphosis. Curr. Biol. 18, 825–830 (2008)
O’Neill, P., McCole, R. B. & Baker, C. V. H. A molecular analysis of neurogenic placode and cranial sensory ganglion development in the shark, Scyliorhinus canicula. Dev. Biol. 304, 156–181 (2007)
Yu, J. K. et al. A cDNA resource for the cephalochordate amphioxus Branchiostoma floridae. Dev. Genes Evol. 218, 723–727 (2008)
Putnam, N. H. et al. The amphioxus genome and the evolution of the chordate karyotype. Nature 453, 1064–1071 (2008)
Gong, S. C., Kus, L. & Heintz, N. Rapid bacterial artificial chromosome modification for large-scale mouse transgenesis. Nature Protocols 5, 1678–1696 (2010)
Villefranc, J. A., Amigo, J. & Lawson, N. D. Gateway compatible vectors for analysis of gene function in the zebrafish. Dev. Dyn. 236, 3077–3087 (2007)
Kawakami, K. et al. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev. Cell 7, 133–144 (2004)
Cameron, T. L., Belluoccio, D., Farlie, P. G., Brachvogel, B. & Bateman, J. F. Global comparative transcriptome analysis of cartilage formation in vivo. BMC Dev. Biol. 9, 20 (2009)
Acknowledgements
We thank D. Brunelle, C.-H. Tung and T.-K. Huang for assistance with amphioxus husbandry, and P. Tsai and D. W. Stock for use of their microtomes. D.M.M., D.J., TA.S. and M.V.C. were supported by National Science Foundation grants IOS 1257040 and IOS 1160733 (D.M.M.) and University of Colorado, Boulder start-up funds (D.M.M.). A.T.G. was supported by National Science Foundation grant DBI 0905991. J.-K.Y. was supported by National Science Council Taiwan grants NSC101-2923-B-001-004-MY2 and NSC102-2311-B-001-011-MY3 and Career Development Award AS-98-CDA-L06 from Academia Sinica Taiwan.
Author information
Authors and Affiliations
Contributions
D.M.M. designed the project and wrote the manuscript. D.J., A.T.G. and T.A.S. performed the experiments and prepared the images; D.J. assembled the figures; and M.V.C. and J.-K.Y. provided materials and reagents. All authors discussed the results and provided input on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Amphioxus oral cartilage differentiation is initiated before cirrus outgrowth and requires FGF-mediated signalling.
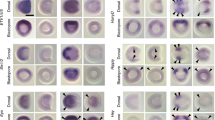
a, b, Phase contrast images of a metamorphic amphioxus larva. a, The differentiation of cartilage rods occurs first in the rim of the mouth. b, The cartilage rods later grow outwards into nascent cirri. c, Toluidine blue staining of a JB-4-embedded control larval section showing the oral cartilage rod (red rectangle; arrows in f) embedded in the rim of the mouth. d, e, Toluidine blue staining of two representative SU5402-treated larvae, sectioned at the same level as the larva in c. The differentiation of the oral cartilage bar is completely eliminated in d and is strongly reduced in e. f–h, High magnification views of the sections in c–e.
Extended Data Figure 2 Expression of SoxE, ColA and FGF signalling components in the oral region of metamorphic amphioxus larvae.
a–l, In situ hybridization was performed on transverse sections in the planes shown in a. b, A high magnification view of cirri hybridized with a probe against ColA. ColA mRNA was detected in the central cartilage rod, which is a single stack of discoidal chondrocytes. c, A high magnification view of cirri hybridized with a probe against SoxE. SoxE mRNA was detected in the central cartilage rod. d, A high magnification view of cirri hybridized with a probe against FGFR. e, f, High magnification views of cirri hybridized with a probe against Ets. g–i, High magnification views of cirri hybridized with a probe against ColA. j, k, High magnification views of cirri hybridized with a probe against DUSP6/7/9. l, A high magnification view of cirri hybridized with a probe against DUSP1/4/5, an orthologue of mouse Dusp4, a gene that is expressed at high levels in mesoderm-derived chondrocytes37. In all panels, the arrows indicate expression in oral chondrocytes, and the arrowheads indicate expression in the associated oral mesothelial cells.
Extended Data Figure 3 Activity of the amphioxus SoxE reporter construct in zebrafish during development.
Injected zebrafish embryos displaying broad GFP fluorescence and normal morphology were processed for in situ hybridization to detect GFP mRNA. Embryos were scored for the expression of GFP in five or more cells in each domain. a, A map of the expression domains scored in 15-somite and 18-somite embryos. b, A representative 4-day larva showing sporadic expression in the heart region and trunk muscles (arrows in insets). The scored domains are outlined. The image is a composite of eight photographs of the same larva taken at different focal planes. c, A representative 18-somite tfap2a and tfap2c dual knockdown morphant (tfap2a/c morphant)28 expressing the amphioxus SoxE reporter. The image is a composite of four photographs of the same embryo taken at different focal planes. The tfap2a/c morphants28 displayed a highly penetrant and almost complete loss of NCC marker expression, including sox10 (a co-orthologue of amphioxus SoxE). Like wildtype embryos, tfap2a/c morphant embryos displayed mosaic expression of the amphioxus SoxE reporter in the neural tube (double arrowheads) and tail bud (arrow). The ability of the amphioxus SoxE reporter to function in tfap2-depleted embryos supports expression data27 suggesting that amphioxus SoxE transcription is Tfap2 independent. d, The numbers and frequencies of embryos and larvae with GFP expression in the indicated domains. The numbers in each column are the pooled results of two to four separate experiments.
Rights and permissions
About this article
Cite this article
Jandzik, D., Garnett, A., Square, T. et al. Evolution of the new vertebrate head by co-option of an ancient chordate skeletal tissue. Nature 518, 534–537 (2015). https://doi.org/10.1038/nature14000
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14000
This article is cited by
-
Cartilage diversification and modularity drove the evolution of the ancestral vertebrate head skeleton
EvoDevo (2023)
-
The Origin and Fate of Chondrocytes: Cell Plasticity in Physiological Setting
Current Osteoporosis Reports (2023)
-
Gene regulatory network from cranial neural crest cells to osteoblast differentiation and calvarial bone development
Cellular and Molecular Life Sciences (2022)
-
Ultrastructural and molecular analysis of the origin and differentiation of cells mediating brittle star skeletal regeneration
BMC Biology (2021)
-
Non-ammocoete larvae of Palaeozoic stem lampreys
Nature (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.