Abstract
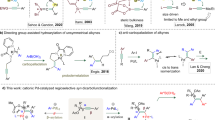
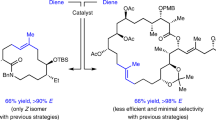
Olefin metathesis catalysts provide access to molecules that are indispensable to physicians and researchers in the life sciences1,2. A persisting problem, however, is the dearth of chemical transformations that directly generate acyclic Z allylic alcohols, including products that contain a hindered neighbouring substituent or reactive functional units such as a phenol, an aldehyde, or a carboxylic acid. Here we present an electronically modified ruthenium–disulfide catalyst that is effective in generating such high-value compounds by cross-metathesis. The ruthenium complex is prepared from a commercially available precursor and an easily generated air-stable zinc catechothiolate. Transformations typically proceed with 5.0 mole per cent of the complex and an inexpensive reaction partner in 4–8 hours under ambient conditions; products are obtained in up to 80 per cent yield and 98:2 Z:E diastereoselectivity. The use of this catalyst is demonstrated in the synthesis of the naturally occurring anti-tumour agent neopeltolide and in a single-step stereoselective gram-scale conversion of a renewable feedstock (oleic acid) to an anti-fungal agent. In this conversion, the new catalyst promotes cross-metathesis more efficiently than the commonly used dichloro–ruthenium complexes, indicating that its utility may extend beyond Z-selective processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hoveyda, A. H. & Zhugralin, A. R. The remarkable metal-catalyzed olefin metathesis reaction. Nature 450, 243–251 (2007)
Fürstner, A. Teaching metathesis “simple” stereochemistry. Science 341, 1357–1364 (2013)
Ibrahem, I., Yu, M., Schrock, R. R. & Hoveyda, A. H. Highly Z- and enantioselective ring-opening/cross-metathesis reactions catalyzed by stereogenic-at-Mo adamantylimido complexes. J. Am. Chem. Soc. 131, 3844–3845 (2009)
Meek, S. J., O’Brien, R. V., Llaveria, J., Schrock, R. R. & Hoveyda, A. H. Catalytic Z-selective olefin cross-metathesis for natural product synthesis. Nature 471, 461–466 (2011)
Yu, M. et al. Synthesis of macrocyclic natural products by catalyst-controlled stereoselective ring-closing metathesis. Nature 479, 88–93 (2011)
Keitz, B. K., Endo, K., Herbert, M. B. & Grubbs, R. H. Z-selective homodimerization of terminal olefins with a ruthenium metathesis catalyst. J. Am. Chem. Soc. 133, 9686–9688 (2011)
Keitz, B. K., Endo, K., Patel, P. R., Herbert, M. B. & Grubbs, R. H. Improved ruthenium catalysts for Z-selective olefin metathesis. J. Am. Chem. Soc. 134, 693–699 (2012)
Rosebrugh, L. E., Herbert, M. B., Marx, V. M., Keitz, B. K. & Grubbs, R. H. Highly active ruthenium metathesis catalysts exhibiting unprecedented activity and Z selectivity. J. Am. Chem. Soc. 135, 1276–1279 (2013)
Occhipinti, G., Hansen, F. R., Törnroos, K. W. & Jensen, V. R. Simple and highly Z-selective ruthenium-based olefin metathesis catalyst. J. Am. Chem. Soc. 135, 3331–3334 (2013)
Khan, R. K. M., Torker, S. & Hoveyda, A. H. Readily accessible and easily modifiable Ru-based catalysts for efficient and Z-selective ring-opening metathesis polymerization and ring-opening/cross-metathesis. J. Am. Chem. Soc. 135, 10258–10261 (2013)
Koh, M. J., Khan, R. K. M., Torker, S. & Hoveyda, A. H. Broadly applicable Z- and diastereoselective ring-opening/cross-metathesis catalyzed by a dithiolate Ru complex. Angew. Chem. Int. Ed. 53, 1968–1972 (2014)
Hoveyda, A. H. Evolution of catalytic stereoselective olefin metathesis. From ancillary transformation to purveyor of stereochemical identity. J. Org. Chem. 79, 4763–4792 (2014)
Mann, T. J., Speed, A. W. H., Schrock, R. R. & Hoveyda, A. H. Catalytic Z-selective cross-metathesis with secondary silyl- and benzyl-protected allylic ethers: mechanistic aspects and applications to natural product synthesis. Angew. Chem. Int. Edn 52, 8395–8400 (2013)
Buchmeiser, M. R., Sen, S., Unold, J. & Frey, W. N-Heterocyclic carbene, high oxidation state molybdenum alkylidene complexes: Functional-group-tolerant cationic metathesis catalysts. Angew. Chem. Int. Edn 53, 9384–9388 (2014)
Lin, Y. A. & Davis, B. G. The allylic chalcogen effect in olefin metathesis. J. Org. Chem. 6, 1219–1228 (2010)
Torker, S., Khan, R. K. M. & Hoveyda, A. H. The influence of anionic ligands on stereoisomerism of Ru carbenes and their importance to efficiency and selectivity of catalytic olefin metathesis reactions. J. Am. Chem. Soc. 136, 3439–3455 (2014)
Werner, H., Grünwald, C., Stüer, W. & Wolf, J. Deactivation of the Grubbs carbene complex [RuCl2( = CHPh)(PCy3)2] by allylic alcohols. Organometallics 22, 1558–1560 (2003)
Hoveyda, A. H., Lombardi, P. J., O’Brien, R. V. & Zhugralin, A. R. H-bonding as a control element in stereoselective Ru-catalyzed olefin metathesis. J. Am. Chem. Soc. 131, 8378–8379 (2009)
Cannon, J. S. & Grubbs, R. H. Alkene chemoselectivity in ruthenium-catalyzed Z-selective olefin metathesis. Angew. Chem. Int. Edn 52, 9001–9004 (2013)
Hartung, J. & Grubbs, R. H. Catalytic, enantioselective synthesis of 1,2-anti-diols by asymmetric ring-opening/cross-metathesis. Angew. Chem. Int. Ed. 53, 3885–3888 (2014)
Rossiter, B. E., Katsuki, T. & Sharpless, K. B. Asymmetric epoxidation provides shortest routes to four chiral epoxy alcohols which are key intermediates in syntheses of methymycin, erythromycin, leukotriene C-1, and disparlure. J. Am. Chem. Soc. 103, 464–465 (1981)
Kiesewetter, E. T. et al. Synthesis of Z-(pinacolato)allylboron and Z-(pinacolato)alkenylboron compounds through stereoselective catalytic cross-metathesis. J. Am. Chem. Soc. 135, 6026–6029 (2013)
Wright, A. E. et al. Neopeltolide, a macrolide from Lithistid sponge of the family Neopeltidae. J. Nat. Prod. 70, 412–416 (2007)
D’Ambrosio, M., Guerriero, A., Debitus, C. & Pietra, F. 6. Leucascandrolide A, a new type of macrolide: the first powerfully bioactive metabolite of calcareous sponges (Leucascandra caveolata, a new genus from the coral sea). Helv. Chim. Acta 79, 51–60 (1996)
Miao. Yu. Schrock, R. R. & Hoveyda, A. H. Catalyst-controlled stereoselective olefin metathesis as a principal strategy in multi-step synthesis design. A concise route to (+)-neopeltolide. Angew. Chem. Int. Ed. http://dx.doi.org/ 10.1002/anie.201409120 (2014)
Biermann, U., Bornscheuer, U., Meier, M. A. R., Metzger, J. & Schäfer, H. Oils and fats as renewable raw materials in chemistry. Angew. Chem. Int. Ed. 50, 3854–3871 (2011)
Gunstone, F. D. in Oleochemical Manufacture and Applications (eds Gunstone, F. D. & Hamilton, R. J. ) Vol. 1 (Academic Press, 2001)
Behr, A. & Gomes, J. P. The cross-metathesis of methyl oleate with cis-2-butene-1,4-diyl acetate and the influence of protecting groups. J. Org. Chem. 7, 1–8 (2011)
Suzuki, Y., Kurita, O., Kono, Y., Hyakutake, H. & Sakurai, A. Structure of a new antifungal C11-hydroxyfatty acid isolated from leaves of wild rice (Oryza officinalis). Biosci. Biotechnol. Biochem. 59, 2049–2051 (1995)
Kajetanowicz, A., Sytniczuk, A. & Grela, K. Metathesis of renewable raw materials—influence of ligands in the indenylidene type catalysts on self-metathesis of methyl oleate and cross-metathesis of methyl oleate with (Z)-2-butene-1,4-diol diacetate. Green Chem. 16, 1579–1585 (2014)
Acknowledgements
This research was supported by a grant from the National Science Foundation (CHE-1362763). R.K.M.K. and M.Y. were partially supported as AstraZeneca Graduate Fellows. We thank Boston College for access to computational facilities.
Author information
Authors and Affiliations
Contributions
M.J.K. and R.K.M.K. carried out the catalyst synthesis, method development studies and applications related to renewable feedstock, S.T. performed the computational investigations, M.Y. carried out the experiments in connection with neopeltolide, and M.S.M. studied modes of catalyst decomposition. A.H.H. conceived and directed the investigations and composed the manuscript with revisions provided by the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data – see contents pages for details. (PDF 11364 kb)
Rights and permissions
About this article
Cite this article
Koh, M., Khan, R., Torker, S. et al. High-value alcohols and higher-oxidation-state compounds by catalytic Z-selective cross-metathesis. Nature 517, 181–186 (2015). https://doi.org/10.1038/nature14061
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14061
This article is cited by
-
Stereocontrolled acyclic diene metathesis polymerization
Nature Chemistry (2023)
-
Structurally defined anti-π-allyliridium complexes catalyse Z-retentive asymmetric allylic alkylation of oxindoles
Nature Catalysis (2022)
-
Toward E-selective Olefin Metathesis: Computational Design and Experimental Realization of Ruthenium Thio-Indolate Catalysts
Topics in Catalysis (2022)
-
Modern Approaches to the Creation of Immobilized Metal-Complex Catalysts for Hydrogenation, Alkene Metathesis, and Cross-Coupling Processes: A Review
Theoretical and Experimental Chemistry (2020)
-
Kinetically E-selective macrocyclic ring-closing metathesis
Nature (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.