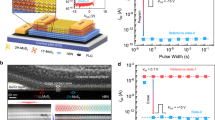
Abstract
Flash memory devices—that is, non-volatile computer storage media that can be electrically erased and reprogrammed—are vital for portable electronics, but the scaling down of metal–oxide–semiconductor (MOS) flash memory to sizes of below ten nanometres per data cell presents challenges. Molecules have been proposed to replace MOS flash memory1, but they suffer from low electrical conductivity, high resistance, low device yield, and finite thermal stability, limiting their integration into current MOS technologies. Although great advances have been made in the pursuit of molecule-based flash memory2, there are a number of significant barriers to the realization of devices using conventional MOS technologies3,4,5,6,7. Here we show that core–shell polyoxometalate (POM) molecules8 can act as candidate storage nodes for MOS flash memory. Realistic, industry-standard device simulations validate our approach at the nanometre scale, where the device performance is determined mainly by the number of molecules in the storage media and not by their position. To exploit the nature of the core–shell POM clusters, we show, at both the molecular and device level, that embedding [(Se(iv)O3)2]4− as an oxidizable dopant in the cluster core allows the oxidation of the molecule to a [Se(v)2O6]2− moiety containing a {Se(v)–Se(v)} bond (where curly brackets indicate a moiety, not a molecule) and reveals a new 5+ oxidation state for selenium. This new oxidation state can be observed at the device level, resulting in a new type of memory, which we call ‘write-once-erase’. Taken together, these results show that POMs have the potential to be used as a realistic nanoscale flash memory. Also, the configuration of the doped POM core may lead to new types of electrical behaviour9,10,11. This work suggests a route to the practical integration of configurable molecules in MOS technologies as the lithographic scales approach the molecular limit12.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
Data deposits
Atomic coordinates for the reported crystal structures have been deposited with the Cambridge Structural Database under the accession codes 997534 (compound precursor), 997535 (compound 1a), 997536 (compound 1c) and 997537 (compound 1d), and full synthetic, electrochemical, device theory, device modelling and electronic device data is given in the Supplementary Information.
References
Joachim, C., Gimzewski, J. K. & Aviram, A. Electronics using hybrid-molecular and mono-molecular devices. Nature 408, 541–548 (2000)
Shaw, J. T. et al. Integration of self-assembled redox molecules in flash memories. IEEE Trans. Electron. Dev. 58, 826–834 (2011)
Zhu, H. et al. Non-volatile memory with self-assembled ferrocene charge trapping layer. Appl. Phys. Lett. 103, 53102–53104 (2013)
Chen, P.-C., Shen, G. & Zhou, C. Chemical sensors and electronic noses based on 1-D metal oxide nanostructures. IEEE Trans. Nanotechnol. 7, 668–682 (2008)
Shaw, J., Xu, Q., Rajwade, S., Hou, T.-H. & Kan, E. C. Redox molecules for a resonant tunneling barrier in nonvolatile memory. IEEE Trans. Electron. Dev. 59, 1189–1198 (2012)
Seol, M.-L., Choi, S.-J., Kim, C.-H., Moon, D.-I. & Choi, Y.-K. Porphyrin-silicon hybrid field-effect transistor with individually addressable top-gate structure. ACS Nano 6, 183–189 (2012)
Tans, S. J., Verschueren, A. R. M. & Dekker, C. Room-temperature transistor based on a single carbon nanotube. Nature 393, 49–52 (1998)
Long, D. L. & Cronin, L. Towards polyoxometalate integrated nanosystems. Chem. Eur. J. 12, 3698–3706 (2006)
Lehmann, J., Gaita-Arino, A., Coronado, E. & Loss, D. Spin qubits with electrically gated polyoxometalate molecules. Nature Nanotechnol. 2, 312–317 (2007)
Li, H. et al. Layer-by-layer assembly and UV photoreduction of graphene–polyoxometalate composite films for electronics. J. Am. Chem. Soc. 133, 9423–9429 (2011)
Fleming, L. et al. Surface-mediated reversible electron transfer reactions within a molecular metal oxide nano-cage. Nature Nanotechnol. 3, 229–233 (2008)
Bonfiglio, V. & Iannaccone, G. Sensitivity-based investigation of threshold voltage variability in 32-nm flash memory cells and MOSFETs. Solid-State Electron. 84, 127–131 (2013)
Vilà-Nadal, L. et al. Polyoxometalate {W18O56XO6} clusters with embedded redox-active main-group templates as localized inner-cluster radicals. Angew. Chem. Int. Ed. 52, 9695–9699 (2013)
Vilà-Nadal, L. et al. Towards polyoxometalate-cluster-based nano-electronics. Chem. Eur. J. 19, 16502–16511 (2013)
Gallon, C. et al. Electrical analysis of mechanical stress induced by STI in short MOSFETs using externally applied stress. IEEE Trans. Electron. Dev. 51, 1254–1261 (2004)
Simmons, J. G. Generalized formula for the electric tunnel effect between similar electrodes separated by a thin insulating film. J. Appl. Phys. 34, 1793–1803 (1963)
Fowler, R. H. & Nordheim, L. Electron emission in intense electric fields. Proc. R. Soc. Lond. A 119, 173–181 (1928)
Acknowledgements
We gratefully acknowledge financial support from the EPSRC for funding (grants EP/H024107/1, EP/I033459/1 and EP/J015156/1), the COST Action CM1203 (PoCheMoN), the Royal Society Wolfson Foundation for a Merit Award, and the University of Glasgow. V.P.G. and A.A. thank S. Markov and S. M. Amoroso for discussions.
Author information
Authors and Affiliations
Contributions
L.C. conceived the idea, designed the project and coordinated the efforts of the research team. J.Y. synthesised the clusters and conducted the first electrochemistry experiments and structural characterization with D.-L.L. H.N.M., C.B., L.V.-N., and L.C. helped to characterize the physical properties of the clusters. C.B. did the electron paramagnetic resonance, electrochemistry and spectroscopic measurements. L.V.-N., L.C., V.P.G. and A.A. designed the theory-to-modelling strategy. L.V.-N., with J.M.P., did the DFT calculations. V.P.G. and A.A. did the device simulation. R.H.P. and N.G. fabricated and characterized the electrode arrays, produced the devices, made the measurements and characterized the data. M.M.M. and D.J.P. designed the nanowire arrays and M.M.M. fabricated the electrodes and optimized the data with D.J.P., who helped analyse the results. C.B., L.V.-N. and L.C. co-wrote the paper with input from all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data, Supplementary Figures 1-14, Supplementary Tables 1-8 and Supplementary References. (PDF 3238 kb)
Rights and permissions
About this article
Cite this article
Busche, C., Vilà-Nadal, L., Yan, J. et al. Design and fabrication of memory devices based on nanoscale polyoxometalate clusters. Nature 515, 545–549 (2014). https://doi.org/10.1038/nature13951
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13951
This article is cited by
-
Heteropoly ionic liquids functionalized γ-Fe2O3 NPs: synthesis, characterization, and catalytic application in selective oxidation of benzyl alcohol to benzaldehyde using H2O2 as a green oxidant
Research on Chemical Intermediates (2023)
-
Room-temperature logic-in-memory operations in single-metallofullerene devices
Nature Materials (2022)
-
Self-assembly of polyoxometalate clusters into two-dimensional clusterphene structures featuring hexagonal pores
Nature Chemistry (2022)
-
Pure Metal Clusters with Atomic Precision for Nanomanufacturing
Nanomanufacturing and Metrology (2022)
-
Assembly of Dy60 and Dy30 cage-shaped nanoclusters
Communications Chemistry (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.