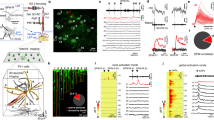
Abstract
Long-term synaptic potentiation (LTP) is thought to be a key process in cortical synaptic network plasticity and memory formation1. Hebbian forms of LTP depend on strong postsynaptic depolarization, which in many models is generated by action potentials that propagate back from the soma into dendrites2,3. However, local dendritic depolarization has been shown to mediate these forms of LTP as well1,4,5. As pyramidal cells in supragranular layers of the somatosensory cortex spike infrequently6,7,8, it is unclear which of the two mechanisms prevails for those cells in vivo. Using whole-cell recordings in the mouse somatosensory cortex in vivo, we demonstrate that rhythmic sensory whisker stimulation efficiently induces synaptic LTP in layer 2/3 (L2/3) pyramidal cells in the absence of somatic spikes. The induction of LTP depended on the occurrence of NMDAR (N-methyl-d-aspartate receptor)-mediated long-lasting depolarizations, which bear similarities to dendritic plateau potentials9,10,11,12,13. In addition, we show that whisker stimuli recruit synaptic networks that originate from the posteromedial complex of the thalamus (POm). Photostimulation of channelrhodopsin-2 expressing POm neurons generated NMDAR-mediated plateau potentials, whereas the inhibition of POm activity during rhythmic whisker stimulation suppressed the generation of those potentials and prevented whisker-evoked LTP. Taken together, our data provide evidence for sensory-driven synaptic LTP in vivo, in the absence of somatic spiking. Instead, LTP is mediated by plateau potentials that are generated through the cooperative activity of lemniscal and paralemniscal synaptic circuitry14,15,16.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bliss, T. V. P. & Collingridge, G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39 (1993)
Markram, H., Luebke, J., Frotscher, M. & Sakmann, B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275, 213–215 (1997)
Feldman, D. E. The spike-timing dependence of plasticity. Neuron 75, 556–571 (2012)
Golding, N. L., Staff, N. P. & Spruston, N. Dendritic spikes as a mechanism for cooperative long-term potentiation. Nature 418, 326–331 (2002)
Lisman, J. & Spruston, N. Postsynaptic depolarization requirements for LTP and LTD: a critique of spike timing-dependent plasticity. Nature Neurosci. 8, 839–841 (2005)
Brecht, M., Roth, A. & Sakmann, B. Dynamic receptive fields of reconstructed pyramidal cells in layers 3 and 2 of rat somatosensory barrel cortex. J. Physiol. (Lond.) 553, 243–265 (2003)
Poulet, J. F. & Petersen, C. C. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature 454, 881–885 (2008)
O'Connor, D. H., Peron, S. P., Huber, D. & Svoboda, K. Neural activity in barrel cortex underlying vibrissa-based object localization in mice. Neuron 67, 1048–1061 (2010)
Antic, S. D., Zhou, W. L., Moore, A. R., Short, S. M. & Ikonomu, K. D. The decade of the dendritic NMDA spike. J. Neurosci. Res. 88, 2991–3001 (2010)
Lavzin, M., Rapoport, S., Polsky, A., Garion, L. & Schiller, J. Nonlinear dendritic processing determines angular tuning of barrel cortex neurons in vivo. Nature 490, 397–401 (2012)
Xu, N. L. et al. Nonlinear dendritic integration of sensory and motor input during an active sensing task. Nature 492, 247–251 (2012)
Major, G., Larkum, M. E. & Schiller, J. Active properties of neocortical pyramidal neuron dendrites. Annu. Rev. Neurosci. 36, 1–24 (2013)
Palmer, L. M. et al. NMDA spikes enhance action potential generation during sensory input. Nature Neurosci. 17, 383–390 (2014)
Bureau, I., von Saint Paul, F. & Svoboda, K. Interdigitated paralemniscal and lemniscal pathways in the mouse barrel cortex. PLoS Biol. 4, e382 (2006)
Diamond, M. E., von Heimendahl, M., Knutsen, P. M., Kleinfeld, D. & Ahissar, E. ‘Where’ and ‘what’ in the whisker sensorimotor system. Nature Rev. Neurosci. 9, 601–612 (2008)
Larkum, M. A cellular mechanism for cortical associations: an organizing principle for the cerebral cortex. Trends Neurosci. 36, 141–151 (2013)
Megevand, P. et al. Long-term plasticity in mouse sensorimotor circuits after rhythmic whisker stimulation. J. Neurosci. 29, 5326–5335 (2009)
Gambino, F. & Holtmaat, A. Spike-timing-dependent potentiation of sensory surround in the somatosensory cortex is facilitated by deprivation-mediated disinhibition. Neuron 75, 490–502 (2012)
Poulet, J. F., Fernandez, L. M., Crochet, S. & Petersen, C. C. Thalamic control of cortical states. Nature Neurosci. 15, 370–372 (2012)
Favero, M. & Castro-Alamancos, M. A. Synaptic cooperativity regulates persistent network activity in neocortex. J. Neurosci. 33, 3151–3163 (2013)
Armstrong-James, M., Welker, E. & Callahan, C. A. The contribution of NMDA and non-NMDA receptors to fast and slow transmission of sensory information in the rat SI barrel cortex. J. Neurosci. 13, 2149–2160 (1993)
Petersen, C. C., Grinvald, A. & Sakmann, B. Spatiotemporal dynamics of sensory responses in layer 2/3 of rat barrel cortex measured in vivo by voltage-sensitive dye imaging combined with whole-cell voltage recordings and neuron reconstructions. J. Neurosci. 23, 1298–1309 (2003)
Wilent, W. B. & Contreras, D. Synaptic responses to whisker deflections in rat barrel cortex as a function of cortical layer and stimulus intensity. J. Neurosci. 24, 3985–3998 (2004)
Rema, V., Armstrong-James, M. & Ebner, F. F. Experience-dependent plasticity of adult rat S1 cortex requires local NMDA receptor activation. J. Neurosci. 18, 10196–10206 (1998)
Grienberger, C., Chen, X. & Konnerth, A. NMDA receptor-dependent multidendrite Ca2+ spikes required for hippocampal burst firing in vivo. Neuron 81, 1274–1281 (2014)
Deschênes, M., Veinante, P. & Zhang, Z. W. The organization of corticothalamic projections: reciprocity versus parity. Brain Res. Brain Res. Rev. 28, 286–308 (1998)
Petreanu, L., Mao, T., Sternson, S. M. & Svoboda, K. The subcellular organization of neocortical excitatory connections. Nature 457, 1142–1145 (2009)
Feldmeyer, D. Excitatory neuronal connectivity in the barrel cortex. Front. Neuroanat. 6, 24 (2012)
Diamond, M. E., Armstrong-James, M., Budway, M. J. & Ebner, F. F. Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus: dependence on the barrel field cortex. J. Comp. Neurol. 319, 66–84 (1992)
Hardie, J. & Spruston, N. Synaptic depolarization is more effective than back-propagating action potentials during induction of associative long-term potentiation in hippocampal pyramidal neurons. J. Neurosci. 29, 3233–3241 (2009)
Kitamura, K., Judkewitz, B., Kano, M., Denk, W. & Hausser, M. Targeted patch-clamp recordings and single-cell electroporation of unlabeled neurons in vivo. Nature Methods 5, 61–67 (2008)
Kole, M. H. & Stuart, G. J. Is action potential threshold lowest in the axon? Nature Neurosci. 11, 1253–1255 (2008)
Ohno, S. et al. A morphological analysis of thalamocortical axon fibers of rat posterior thalamic nuclei: a single neuron tracing study with viral vectors. Cereb. Cortex 22, 2840–2857 (2012)
Zhang, F., Wang, L. P., Boyden, E. S. & Deisseroth, K. Channelrhodopsin-2 and optical control of excitable cells. Nature Methods 3, 785–792 (2006)
Akerboom, J. et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 32, 13819–13840 (2012)
Holtmaat, A. et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nature protocols 4, 1128–1144 (2009)
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013)
Pologruto, T. A., Sabatini, B. L. & Svoboda, K. ScanImage: Flexible software for operating laser-scanning microscopes. Biomed. Eng. Online 2, 13 (2003)
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nature Methods 9, 676–682 (2012)
Kapur, J. N., Sahoo, P. K. & Wong, A. K. C. A new method for gray-level picture thresholding using the entropy of the histogram. Computer Vis. Graph. Image Process. 29, 273–285 (1985)
Paxinos, G. & Franklin, K. B. J. The Mouse Brain in Stereotaxic Coordinates. 2nd edn (Academic Press, 2001)
Acknowledgements
We thank E. Ahissar and T. Oram for advice on the muscimol experiments. We appreciate C. Lüscher’s comments on our manuscript. We thank L. L. Looger and D. Kim of the GENIE project, and K. Svoboda at the Janelia Farm Research Campus (HHMI) for distributing the GCaMP5G, GCaMP6S and ChR2 vectors, respectively. This work was supported by the Swiss National Science Foundation (grants 31003A_120685, 31003A_135631 and CRSI33_127289 to A.H.; 31003A_153410 to A.C.), the National Centre of Competence in Research (NCCR) SYNAPSY financed by the Swiss National Science Foundation (51AU40_125759), the International Foundation for Research on Paraplegia, and the Hans Wilsdorf Foundation. V.K. was supported by SyMBaD (EU FP7-PEOPLE-ITN Marie Curie, grant 238608).
Author information
Authors and Affiliations
Contributions
F.G. performed the in vivo electrophysiology experiments; S.P. performed the Ca2+ imaging experiments; V.K. and F.G. performed the photostimulation experiments; D.B. and V.K. performed thalamocortical projection analyses; R.T. and F.G. performed in vitro electrophysiology experiments; A.C. and A.H. provided equipment and technical expertise; F.G., S.P., V.K. and A.H. conceived the studies; A.H. supervised the research; A.H., F.G. and S.P. wrote the manuscript with help from V.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 An NMDAR block suppresses RWS-induced sustained depolarization and prevents LTP.
a, Examples of the postsynaptic depolarization as induced by RWS in controls (top), following a blockade of NMDARs by DAP5 (middle), and hyperpolarization (bottom). Only the first 2.5 s (20 deflections) of the recordings are shown. Responses were fit with an exponential, in which V(t) is the depolarization at time point t in seconds, Vc is the depolarization constant (for example, reached after >10 s RWS), V0 and t0 are the depolarization and time at RWS onset, and τ is the time constant. b, Left, under control conditions (dark blue), the level of LTP is linearly correlated to the time constant (τ) of the exponential decay (R2 = 0.49, P < 0.05). Following a suppression of NMDAR conductances the time constant and the percentage of LTP are independent (DAP5, light blue, R2 = 0.03, P > 0.05; Hyper, red, R2 = 0.03, P > 0.05). Each circle represents a single cell. Right, DAP5 significantly reduced the time constant (τ) of the exponential decay (P < 0.001; Kruskal–Wallis one-way ANOVA on ranks. *P < 0.05, post-hoc Dunn’s comparisons versus control condition). c, Left, the sustained depolarization during RWS is altered when NMDAR conductances are suppressed by hyperpolarization (red). Black bar indicates the RWS period. Right, cumulative depolarization at the end of the RWS period (control, 127 ± 21 mV, n = 11; +DAP5, 41 ± 28 mV, n = 7; Hyper, 34 ± 25 mV, n = 9; P = 0.02, one-way ANOVA (β = 0.62) and *P < 0.05, post-hoc Holm-Sidak comparisons versus control condition). d, RWS failed to induce LTP when NMDARs are blocked by extracellular application of DAP5, when MK801 is included in the patch pipette, and when cells are hyperpolarized (control, 119.8 ± 6%, n = 11; DAP5, 98.7 ± 2.5, n = 7; iMK801, 98 ± 5%, n = 3; Hyper, 103.6 ± 6%, n = 9; P = 0.032, one-way ANOVA and *P < 0.05, post-hoc Holm-Sidak comparisons versus control condition). e, A fast Fourier transform (FFT) of the responses during RWS (1 min), in controls (left) and after DAP5 application (middle). The FFT is normalized to the average FFT between 0.1 and 1 Hz. The presence of a strong 8 Hz component after DAP5 indicates that RWS-mediated inputs remain to be activated after the NMDAR block. Right, the magnitude of normalized FFT at 8 Hz is similar between control +DAP5 conditions (control, 8.8 ± 8.5, n = 11; +DAP5, 6.3 ± 4.6, n = 7; P = 0.733, Mann–Whitney U-test) confirming that part of the whisker deflection-evoked synaptic input was unaffected by DAP5, and follows the rhythmic stimulation. Values in b–e are represented as the mean ± s.e.m.
Extended Data Figure 2 RWS-induced LTP is column- and whisker-specific.
a, Left, schematic of the experiment: whole cell recordings are targeted to the C2 barrel column. Responses are recorded upon deflection of the C2 (principal whisker, wC2) or C1 (surrounding whisker, wC1) whisker. Inset, C2 (blue) and C1 (red) barrel-related columns were mapped using intrinsic optical imaging. Right, schematic of PW and SW-associated synaptic pathways projecting to L2/3 pyramidal cells. After RWS of wC1 (orange), single-whisker deflection PSPs were evoked either by the same whisker that was used for RWS (wC1, red) or by the neighbouring whisker (wC2, blue). b, Time course of mean wC2- (blue) and wC1-evoked (red) PSPshort amplitudes ( ± s.e.m.) following RWS of wC1 (orange bar). c, Mean PSPshort amplitude ( ± s.e.m.) before and after RWS. Top, wC1-RWS did not significantly enhance wC2-evoked mean PSPshort amplitudes (Pre, 7.8 ± 1.1 mV; Post, 7.9 ± 1.3 mV; n = 8; P = 0.960, paired t-test (β = 0.05)). Bottom, wC1-evoked amplitudes were enhanced in some cells, but despite this positive trend the average difference was not significant (Pre, 7.4 ± 1.3 mV; Post, 8.6 ± 1.3 mV; n = 5; P = 0.240, paired t-test (β = 0.15)). Grey lines indicate pairs. d, e, Although the average PSP amplitude as evoked by wC1 was not significantly different from wC2 (wC2, 101 ± 2.5%, n = 8; wC1, 118 ± 10%, n = 5; P = 0.2, Mann–Whitney U-test; d), the number of significantly potentiated cells was higher for wC1 (3 out of 5) than for wC2 (0 out of 9; e).
Extended Data Figure 3 Characterization of RWS-induced LTP and stability of cell membrane properties.
a, For each potentiated cell, a single exponential was fit to the normalized PSPshort amplitudes immediately following RWS, using the following equation: PSP(t) = (1 + ΔPSPLTP) – (ΔPSPLTP · e−(t/τ)), in which PSP(t) is the normalized PSP amplitude at time t in minutes, ΔPSPLTP is the average change in PSP amplitude during the LTP phase, and τ the time constant (mean τ = 1.09 ± 0.26 min; range 0.3–2.34 min; n = 7). b, Left, time course of mean PSPshort amplitudes following two consecutive RWS protocols (RWS1 and RWS2). Right, the mean PSPshort amplitude increases upon RWS1 but does not further increase upon RWS2, indicating that RWS-evoked LTP is occluded (Pre, 10.32 ± 1.6 mV; RWS1, 12.35 ± 1.6 mV; RWS2, 12.98 ± 1.5 mV; n = 5; P = 0.006, repeated measures ANOVA (β = 0.97); post-hoc Holm-Sidak comparisons). The error bars represent s.e.m. c, Example of typical single-cell membrane potential fluctuations during anaesthesia before (top, grey) and after (bottom, black) RWS. Spontaneous action potentials (APs) are rare and visible only during up states. d, Example of spontaneous APs before (grey) and after (black) RWS. APs were time-aligned to their respective threshold. e, Effect of RWS on AP thresholds, in cells that displayed some spontaneous APs before and after RWS (but not during RWS). AP threshold was computed as the minimal membrane potential value at the time corresponding to the peak of the third derivative of the membrane potential. The Δthreshold was calculated as the difference between the mean AP threshold after RWS and the mean AP threshold before RWS (each circle represents a cell). The level of LTP is independent of Δthreshold (R2 = 0.07, P > 0.05, all cells pooled). f, The mean threshold for spontaneous AP is not affected by RWS (Pre, −43.7 ± 0.9 mV; Post, −44.6 ± 1.2 mV; n = 12; P = 0.2, paired t-test (β = 0.13)). g, RWS does not affect the resting membrane potential measured at I = 0 during down states (Pre, −71.5 ± 0.4 mV; Post, −71.4 ± 0.6 mV; n = 12; P = 0.8, paired t-test (β = 0.05)). h, The difference between input resistance (ΔRin) before and after RWS. ΔRin is independent of the level of LTP (R2 = 10−3, P > 0.05; each circle represents a cell). Inset, Rin before (grey) and after (dark blue) RWS are estimated by measuring the steady-state resistance of a hyperpolarizing current pulse. i, The mean Rin is not affected by RWS (Pre, 46.6 ± 7 MΩ; Post, 50 ± 8 MΩ; n = 11; P = 0.13, paired t-test (β = 0.22)). j, The number of evoked APs as a function of injected somatic current injection is not significantly modified by RWS (two-way ANOVA, P > 0.5, n = 4). k, The relationship between ΔRin and the relative change in amplitude of PSPshort. Each circle represents a cell that was hyperpolarized only during RWS (RWS + Hyper, R2 = 0.6). l, The mean Rin is not affected by RWS + Hyper (Pre, 37.4 ± 4 MΩ; Post, 37.7 ± 5 MΩ; n = 9; P = 0.9, paired t-test (β = 0.05)). m, n, The PSPshort amplitude evoked by low frequency (0.1 Hz) single whisker deflections, before (Pre), during (Hyper) and after (Post) hyperpolarization (−100 mV). Hyperpolarization increases the PSP amplitude (m) due to an enhanced driving force (Pre, 11.3 ± 2.5 mV (average −3 to −1 min); Hyper, 14.7 ± 3 mV; Post (average +1 to +2 min), 11.3 ± 2.4 mV; n = 4; P = 0.002, one-way repeated-measures ANOVA (β = 0.98); ***P < 0.001, Holm-Sidak post-hoc comparisons Hyper versus Pre and Post conditions. However, the integrated PSP is significantly reduced due to the absence of plateau potentials (n) (Pre, 0.625 ± 0.2 V·ms; Hyper, 0.335 ± 0.1 V·ms; Post, 0.626 ± 0.2 V·ms; n = 4; P = 0.011, one-way repeated-measures ANOVA (β = 0.86); **P = 0.007, Holm-Sidak post-hoc comparisons Hyper versus Pre and Post conditions).
Extended Data Figure 4 The extraction of whisker-evoked plateau potentials.
a, Individual PSPs (grey lines) and the average PSP (dark blue line) in a single cell in response to single principal whisker deflections (100-ms deflections). Individual traces show short and long-latency components. The responses to the 30 successive deflections reveal two whisker-evoked PSP populations: one that only contains short-latency PSPs and a second population that contains both short and long-latency PSPs. b, Similar to non-spiking cells, the distribution of whisker-evoked actions potentials (APs) also reveals two populations of spikes, based on their onset delay. Inset, for each spiking cell, whisker-evoked spikes were sorted as early and late spikes, according the delay of the first peak of the subthreshold response. The corresponding probabilities were then computed (early, P = 0.04 ± 0.02, n = 15; late, P = 0.06 ± 0.02, n = 15; P = 0.345, z-test). The equal probabilities indicate that L2/3 cells spike as often upon a long-latency depolarization as in response to a short-latency PSP. c, Left, for each trial, the relationship between the PSP half-peak amplitude and the average membrane potential between 50 and 100 ms after the onset reveals two distinct clusters. Dotted line represents the identity line. Right, cluster 1 (top) is defined by an index < 0 and consists of PSPs containing only a short latency PSP that quickly returns to the resting membrane potential. Cluster 2 (bottom) is defined by an index > 0 and consists of compound PSPs with short and long-latency components. The long-latency component of the PSP has a strong plateau-like appearance. Therefore, we defined this index as the Indexplateau. d, Example of the distribution of clusters 1 and 2 over time. The Indexplateau was computed from the example shown in a. The probability of eliciting plateau potentials in a neuron is calculated by dividing the number of PSPs in cluster 2 (N2) by the total number of PSPs (N1 + N2). e, f, Blocking NMDARs (light blue) significantly reduced the probability of eliciting plateau potentials as compared to controls (dark blue) (e, ks test, P < 0.001), but does not affect the amplitude of the short-latency PSP (PSPshort) (f, ks test, P > 0.05). g, Blocking NMDARs by epidural application of DAP5 or intracellular MK801 does not affect the amplitude of PSPshort (control, 10.5 ± 0.8 mV, n = 33; DAP5, 9.5 ± 1.5 mV, n = 12; iMK801, 10.3 ± 1.3 mV, n = 10; P > 0.05, one-way ANOVA (β = 0.05)). h–j, Changes in the length of the whisker deflection period (stimulus length) do not affect the whisker-evoked mean PSPshort amplitude (h), the mean PSP integral (i), or the probability to elicit plateau potentials (j) (n = 4, P > 0.05, one-way ANOVA (β = 0.05)). Values in b and g–j are represented as the mean ± s.e.m.
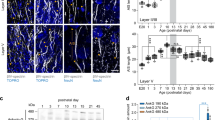
Extended Data Figure 5 NMDAR-dependent whisker-evoked Ca2+ events in L2/3 pyramidal neuron dendritic tufts.
a, Examples of single whisker deflection-evoked Ca2+ responses in dendritic spines (arrowheads) and dendritic shafts (regions in between arrows). b, Top left, GCaMP6 s fluorescence standard deviation image with ROIs. Bottom left, raster plot of ΔF/F0 for each ROI in the top panel (aligned). Red bar represents the whisker stimulation onset. Right, ΔF/F0 traces of some ROIs from a. c, Left, time course of mean dendritic Ca2+ response in individual dendritic branches upon a single whisker deflection (2–5 trials per branch, n = 48 dendritic branches, n = 9 mice). Grey, individual branches. Black, average response. Right, distribution of response onset times. d, Averaged (blue thick line) and individual (grey lines) Gaussian fits of local responsive regions in dendritic shafts. e, Distribution of the FWHM of the Guassian fits in d. f, Epidural application of DAP5 (10 µM) significantly reduces whisker-evoked local dendritic Ca2+ response probabilities. The subsequent addition of 1 mM DAP5 in some cases further reduced probabilities and in others did not show an additive effect (control, 0.29 ± 0.06, DAP5 (10 µM), 0.14 ± 0.04; n = 16 branches, n = 3 mice; P = 0.002, Wilcoxon signed-rank test; DAP5 (10 µM), 0.08 ± 0.03; DAP5 (1 mM), 0.04 ± 0.02; n = 9 branches, N = 2 mice; P = 0.37, Wilcoxon signed-rank test). g, On average, DAP5 significantly reduces whisker-evoked local dendritic Ca2+ response probabilities (control, 0.28 ± 0.03; DAP5 (10 µM), 0.12 ± 0.03; DAP5 (1 mM), 0.04 ± 0.02; Kruskal–Wallis one-way ANOVA on ranks; post-hoc Dunn’s comparisons versus control condition, P < 0.05)). h, Left, GCaMP6 s fluorescence standard deviation image with ROIs. Right, ΔF/F0 traces of some ROIs from the left panel. Grey box represents the RWS period. i, Integrated ΔF/F0 in dendritic branches during RWS (0–15 s) as a function of the response before RWS (0–15 s baseline). Each circle represents a single dendritic branch. Red, global events (responses spanning the whole field of view, minimally 43 µm); grey, local events (responses spanning a portion of the field of view, maximally 43 µm). Black line indicates the identity line. RWS significantly increases ΔF/F0 for a substantial number of branches. j, The average integrated ΔF/F0 in dendritic branches during RWS (0–15 s) is significantly reduced upon topical application of DAP5 (control, 168 ± 15%; DAP5 (10 µM), 119.6 ± 14.5%; DAP5 (1mM), 100.1 ± 18.5%; paired t-test).
Extended Data Figure 6 Whisker-evoked activity in cortical POm efferents in anaesthetized and awake mice.
a, c, Raster plots of average Ca2+ responses (ΔF/F0) in 400 different cortical POm efferent boutons over 10–20 successive (0.1 Hz) bursts of 5 whisker deflections (20 Hz, black bar) in anesthetized mice (a) and in awake mice (c). b, d, Average whisker-evoked responses over 400 boutons under anaesthesia (b) and under wakefulness (d). e, Raster plot for 25 of the most active boutons (out of 765) upon RWS. Black bars indicate the period of RWS (1 min). The Ca2+ response in some boutons remains elevated over the whole RWS period. f, Integrated Ca2+ responses of individual boutons during RWS (0–15 s) as a function of their responses before RWS (15 s baseline). Each circle represents a single bouton. The 25 most and least responsive boutons are in blue and red respectively. Black line indicates the mean relationship (linear fit). Dotted lines indicate the relationship at various standard deviations from the fit. A substantial proportion of boutons (16%; 124 out of 765) display RWS/baseline ratios larger than 1 s.d. from the mean. g, Time-lapse image of fluorescence change representing Ca2+ responses in axonal boutons (dotted circles) upon a single whisker deflection (red bar; 45 ms). Response onsets are indicated by arrows. Scale bar represents 1 μm. h, Example of the response curve of the boutons in g. Response onset latency was defined as the time frame in which ΔF/F0 exceeded 2 × s.d. of the baseline. i, j, Distributions of response onset latencies under anaesthesia (black, 120 trials, n = 5 boutons, n = 3 mice) and under wakefulness (blue, 11 trials, n = 5 boutons, n = 3 mice).
Extended Data Figure 7 Plateau potentials in L2/3 pyramidal neurons and POm-efferent activity are not whisker-specific.
a, Schematic of the experiment. Whole-cell recordings are targeted to the C2 barrel column. Responses are recorded upon deflection of the C2 whisker (wC2, principal whisker, PW, blue) or the C1 whisker (wC1, surrounding whisker, SW, red). b, Right, example of 2PLSM images of POm boutons expressing GCaMP5. Both the PW (wC2) and SW (wC1) evoke a Ca2+ response. c, Left, the average Ca2+ transient (ΔF/F0) for both whisker deflections (shadows represent the s.d.). Right, mean ΔF/F0 upon deflection of the PW (wC2) and SW (wC1) (wC2, 1.37 ± 0.07, n = 5; wC2, 1.38 ± 0.08, n = 5; P > 0.05). This confirms that POm activity is not selective for whiskers. Values are represented as mean ± s.e.m. d, Top, example of the average PSP evoked by the PW (blue) or the SW (red). To estimate the integral of the plateau potential (bottom), the decay of the first component is fitted with a single exponential and subtracted from the average of PSPs containing both short and late-latency components (cluster 2 in Extended Data Fig. 4c). e, For each parameter, whisker selectivity is defined by the ratio between the PW and SW: (PW − SW)/(PW + SW). All parameters related to plateau potentials (plateau strength, plateau integral, probability) are not specific to either one of the whiskers. In contrast, and as expected, the short-latency PSP amplitude (PSPshort) is higher (and thus more selective) for the PW (PSPshort, 0.25 ± 0.09, n = 27; plateau strength, 0.006 ± 0.05, n = 26; plateau integral, 0.04 ± 0.02, n = 26; plateau probability, 0.07 ± 0.04; n = 27; P < 0.001, one-way ANOVA on ranks; *P < 0.05, post-hoc Dunn’s comparisons versus PSPshort condition). As the amplitude of short-latency PSPs is whisker-selective and plateau potentials are not, it is conceivable that POm associated synaptic pathways are responsible for mediating whisker-evoked plateau potentials.
Extended Data Figure 8 AAV-mediated expression of ChR2-Venus in the POm nuclei of the thalamus and their efferents in L1.
a, Representative example of the ChR2-Venus expression profile in the mouse thalamus. The expression profile of ChR2-Venus is depicted in blue, POm nuclei in light green, and the VPm in light red. b, Left, example of a cortical slice with ChR2-Venus fluorescence in the caudal sector of the POm (bregma −2.3). Right, an image of the fluorescence profile in the somatosensory cortex in the same animal as on the left. Fluorescence intensities (Fint) were measured as a function of cortical depth (in pixels (px)) by summing all pixels within the dotted rectangle over the short axis (pixel size = 1.85 µm). The fluorescence intensity profile is similar to the cortical projection pattern of efferents from the caudal sector of the POm (Supplementary Note 4). c, Plot comparing the intensity profiles of 10 different animals in which injections were aimed at the POm nuclei in the thalamus. Animals classified as bearing expression profiles in the barrel cortex that are typical of POm projections (Supplementary Notes 4 and 5) displayed distinct fluorescence peaks in L1 (0.04 – 0.12 normalized depth) and L5 (0.48 – 0.7 normalized depth; n = 7, black lines and blue line). In contrast, animals with a (additional) distinct peak in L4 (0.36 – 0.48 normalized depth; n = 3, red lines) were considered as having at least some spurious expression in VPm, and were thus excluded from the analysis in Fig. 3. The classification matched the expression profiles in the thalamus (Supplementary Information). d, e, Assessment of POm mediated synaptic inputs onto L2/3 neurons in acute cortical slice preparations. e, Schematic of the slice experiment. L2/3 pyramidal neurons were recorded during local photostimulation (through the objective) of ChR2-expressing POm axons. f, Left, example of photostimulation-evoked PSPs in a single L2/3 cell under control conditions and following bath application of TTX and 4AP. Right, average PSP amplitudes in controls and after TTX + 4AP application (control, 1.8 ± 1.1; TTX + 4AP, 0.31 ± 0.08, n = 4, P = 0.125, paired Wilcoxon signed-rank test).
Extended Data Figure 9 The spread of fluorescent muscimol in thalamic nuclei.
a, Coronal diagrams of the mouse brain adapted from the Paxinos atlas41 including the POm (light green) and VPm (light red) nuclei at various posterior distances from bregma. Each red or green line represents the maximal spread of fluorescent muscimol, as assessed using whole field epifluorescence microscopy (Olympus; ×20 and ×60 objective) and Neurolucida (Microbrightfield) reconstructions. Green lines (8 mice) represent injections that were confined to the POm. Red lines (3 mice) represent injections that infiltrated both POm and VPm thalamic nuclei. b, c, Examples of a muscimol injection in the caudal part of the POm (b) and an injection that spread into both POm and VPm nuclei (c). d, Examples of whisker-evoked PSPs in L2/3 neurons of mice in which muscimol was present in both POm and VPm. Successes (top) and failures (bottom) are shown. Grey lines, individual trials, light red lines, average. e, Blocking activity in VPm decreases the whisker-evoked PSP success rate (top; control, 1 ± 0, n = 33; POm block, 1 ± 0, n = 9; POm + VPm block, 0.56 ± 0.1, n = 7; P < 0.001, one-way ANOVA on ranks; *P < 0.05, post-hoc Dunn’s comparisons versus control condition), as well as PSPshort amplitudes (bottom; control, 10.5 ± 0.8, n = 33; POm block, 7.5 ± 1.3, n = 9; POm + VPm block, 3.15 ± 0.6, n = 7; P < 0.001, one-way ANOVA; *P < 0.05, post-hoc Holm-Sidak’s comparisons versus control condition). f, Blocking POm + VPm or POm only significantly decreases the probability of plateau potentials (top; control, 0.35 ± 0.04, n = 33; POm block, 0.16 ± 0.04, n = 9; VPm block: 0.22 ± 0.05, n = 7; P < 0.001, one-way ANOVA on ranks; *P < 0.05, post-hoc Dunn’s comparisons versus control condition), and the normalized plateau strength (bottom; control, 1 ± 0.15, n = 33; POm block, 0.19 ± 0.05, n = 9; POm + VPm block, 0.22 ± 0.07, n = 7; P < 0.001, one-way ANOVA on ranks; *P < 0.05, post-hoc Dunn’s comparisons versus control condition). g, examples of single-cell spontaneous membrane potential fluctuations during anaesthesia in controls (top) and upon muscimol injections in POm (middle) or POm + VPm (bottom). h, Top left, FFT of membrane potentials in controls (dark blue) and after muscimol injection in POm (green) or POm + VPm (light red). Top right, Blocking POm + VPm significantly decreases the 1–5-Hz range in the FFT (control, 7.33 ± 0.76, n = 14; POm block, 8.03 ± 0.81, n = 8; VPm block, 4.2 ± 0.37, n = 7; P = 0.008, one-way ANOVA; *P < 0.05, post-hoc Holm-Sidak’s comparisons versus control condition). Bottom, blocking POm + VPm significantly decreases the probability of spontaneous up states (left (Pup); control, 0.16 ± 0.016, n = 14; POm block, 0.15 ± 0.008, n = 8; POm + VPm block, 0.09 ± 0.016, n = 7; P = 0.023, one-way ANOVA; *P < 0.05, post-hoc Holm-Sidak’s comparisons versus control condition), as well as the amplitude of spontaneous up states (right (AMPup); control, 22.3 ± 1.9, n = 14; POm block, 21.5 ± 1.6, n = 8; POm + VPm block, 14.1 ± 1.7, n = 7; P = 0.02, one-way ANOVA; *P < 0.05, post-hoc Holm-Sidak’s comparisons versus control condition). i, Animals in which muscimol injections in the medial posterior thalamus did not infiltrate the POm (out of POm) were used as a negative controls (n = 6). In these animals, the probability of eliciting plateau potentials (Pplateau) remained equal to controls. The probability was significantly reduced, only when muscimol was correctly targeted to POm (control, 0.6 ± 0.04, n = 44; +muscimol In POm, 0.16 ± 0.04, n = 9; +muscimol Out POm, 0.72 ± 0.06, n = 8; P < 0.001, one-way ANOVA on ranks; *P < 0.05, post-hoc Dunn’s comparisons versus control condition). The values represent the mean ± s.e.m.
Supplementary information
Supplementary Information
This file contains Supplementary Notes 1-7, Supplementary Valies and Statistics, Supplementary References and Supplementary Data Figure 1. (PDF 943 kb)
Rights and permissions
About this article
Cite this article
Gambino, F., Pagès, S., Kehayas, V. et al. Sensory-evoked LTP driven by dendritic plateau potentials in vivo. Nature 515, 116–119 (2014). https://doi.org/10.1038/nature13664
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13664
This article is cited by
-
Electric field effects on neuronal input–output relationship by regulating NMDA spikes
Cognitive Neurodynamics (2024)
-
The plasticitome of cortical interneurons
Nature Reviews Neuroscience (2023)
-
Excitation–transcription coupling, neuronal gene expression and synaptic plasticity
Nature Reviews Neuroscience (2023)
-
How deep is the brain? The shallow brain hypothesis
Nature Reviews Neuroscience (2023)
-
Coenzyme Q10 supplementation improves the motor function of middle-aged mice by restoring the neuronal activity of the motor cortex
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.