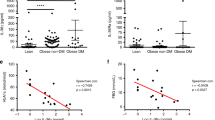
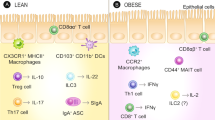
Abstract
The connection between an altered gut microbiota and metabolic disorders such as obesity, diabetes, and cardiovascular disease is well established1,2. Defects in preserving the integrity of the mucosal barriers can result in systemic endotoxaemia that contributes to chronic low-grade inflammation, which further promotes the development of metabolic syndrome3,4,5. Interleukin (IL)-22 exerts essential roles in eliciting antimicrobial immunity and maintaining mucosal barrier integrity within the intestine6,7. Here we investigate the connection between IL-22 and metabolic disorders. We find that the induction of IL-22 from innate lymphoid cells and CD4+ T cells is impaired in obese mice under various immune challenges, especially in the colon during infection with Citrobacter rodentium. While innate lymphoid cell populations are largely intact in obese mice, the upregulation of IL-23, a cytokine upstream of IL-22, is compromised during the infection. Consequently, these mice are susceptible to C. rodentium infection, and both exogenous IL-22 and IL-23 are able to restore the mucosal host defence. Importantly, we further unveil unexpected functions of IL-22 in regulating metabolism. Mice deficient in IL-22 receptor and fed with high-fat diet are prone to developing metabolic disorders. Strikingly, administration of exogenous IL-22 in genetically obese leptin-receptor-deficient (db/db) mice and mice fed with high-fat diet reverses many of the metabolic symptoms, including hyperglycaemia and insulin resistance. IL-22 shows diverse metabolic benefits, as it improves insulin sensitivity, preserves gut mucosal barrier and endocrine functions, decreases endotoxaemia and chronic inflammation, and regulates lipid metabolism in liver and adipose tissues. In summary, we identify the IL-22 pathway as a novel target for therapeutic intervention in metabolic diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tremaroli, V. & Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 489, 242–249 (2012)
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006)
Gregor, M. F. & Hotamisligil, G. S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445 (2011)
Kanneganti, T. D. & Dixit, V. D. Immunological complications of obesity. Nature Immunol. 13, 707–712 (2012)
Vijay-Kumar, M. et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328, 228–231 (2010)
Ouyang, W., Rutz, S., Crellin, N. K., Valdez, P. A. & Hymowitz, S. G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 29, 71–109 (2011)
Colonna, M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity 31, 15–23 (2009)
Myers, M. G., Cowley, M. A. & Munzberg, H. Mechanisms of leptin action and leptin resistance. Annu. Rev. Physiol. 70, 537–556 (2008)
Zheng, Y. et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nature Med. 14, 282–289 (2008)
Sugimoto, K. et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 118, 534–544 (2008)
Sonnenberg, G. F. et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science 336, 1321–1325 (2012)
Guo, X. et al. Leptin signaling in intestinal epithelium mediates resistance to enteric infection by Entamoeba histolytica. Mucosal Immunol. 4, 294–303 (2011)
Van Maele, L. et al. TLR5 signaling stimulates the innate production of IL-17 and IL-22 by CD3negCD127+ immune cells in spleen and mucosa. J. Immunol. 185, 1177–1185 (2010)
Procaccini, C., Jirillo, E. & Matarese, G. Leptin as an immunomodulator. Mol. Aspects Med. 33, 35–45 (2012)
Lord, G. M. et al. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394, 897–901 (1998)
De Rosa, V. et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity 26, 241–255 (2007)
Yu, Y. et al. Leptin-induced RORγt expression in CD4+ T cells promotes Th17 responses in systemic lupus erythematosus. J. Immunol. 190, 3054–3058 (2013)
Maaser, C. et al. Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infect. Immun. 72, 3315–3324 (2004)
Tilg, H. & Kaser, A. Gut microbiome, obesity, and metabolic dysfunction. J. Clin. Invest. 121, 2126–2132 (2011)
Han, M. S. et al. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science 339, 218–222 (2013)
Uysal, K. T., Wiesbrock, S. M., Marino, M. W. & Hotamisligil, G. S. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 389, 610–614 (1997)
Ventre, J. et al. Targeted disruption of the tumor necrosis factor-α gene: metabolic consequences in obese and nonobese mice. Diabetes 46, 1526–1531 (1997)
Hirosumi, J. et al. A central role for JNK in obesity and insulin resistance. Nature 420, 333–336 (2002)
Zhang, X. et al. Selective inactivation of c-Jun NH2-terminal kinase in adipose tissue protects against diet-induced obesity and improves insulin sensitivity in both liver and skeletal muscle in mice. Diabetes 60, 486–495 (2011)
Batterham, R. L. et al. Gut hormone PYY3–36 physiologically inhibits food intake. Nature 418, 650–654 (2002)
Batterham, R. L. et al. Inhibition of food intake in obese subjects by peptide YY3–36 . N. Engl. J. Med. 349, 941–948 (2003)
Ortiz, A. A. et al. A novel long-acting selective neuropeptide Y2 receptor polyethylene glycol-conjugated peptide agonist reduces food intake and body weight and improves glucose metabolism in rodents. J. Pharmacol. Exp. Ther. 323, 692–700 (2007)
Yang, L. et al. Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J. Hepatol. 53, 339–347 (2010)
Odegaard, J. I. et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 (2007)
Zechner, R. et al. Fat signals - lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 15, 279–291 (2012)
Zheng, Y. et al. Interleukin-22, a Th17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445, 648–651 (2007)
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959)
Rosen, E. D. et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 4, 611–617 (1999)
Ota, N. et al. IL-22 bridges the lymphotoxin pathway with the maintenance of colonic lymphoid structures during infection with Citrobacter rodentium. Nature Immunol. 12, 941–948 (2011)
Wu, A. L. et al. Amelioration of type 2 diabetes by antibody-mediated activation of fibroblast growth factor receptor 1. Sci. Translat. Med. 3, 113ra126 (2011)
Barman, M. et al. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect. Immun. 76, 907–915 (2008)
Sham, H. P. et al. SIGIRR, a negative regulator of TLR/IL-1R signalling promotes microbiota dependent resistance to colonization by enteric bacterial pathogens. PLoS Pathog. 9, e1003539 (2013)
Acknowledgements
We acknowledge S. Haller for her work in pathological analysis of colon samples. We thank F. Chu and J. Eastham-Anderson for performing the immunohistochemical staining and analysing pancreatic cell staining.
Author information
Authors and Affiliations
Contributions
W.O. and G.K. devised the project. X.W. and N.O. designed and performed most of the experiments and analyses. P.M. performed western blot and contributed to lipid metabolism studies. G.K., L.K., J.Z.-S., and J.R. contributed to metabolic studies. C.E. and J.L. contributed to flagellin stimulation studies. J.Z. and W.P.L. contributed to ovalbumin/complete Freund’s adjuvant stimulation studies. L.D. did all histology analyses. W.O., X.W., and N.O. prepared the manuscript. N.v.B., P.M., C.E., and G.K. helped to edit the manuscript.
Corresponding authors
Ethics declarations
Competing interests
All authors are employees of Genentech Inc.
Extended data figures and tables
Extended Data Figure 1 Obese mice have defective IL-22 and IL-17A production.
a–c, ELISA of IL-22 in serum 2 h after flagellin injection from db/db and lean control mice (a), ob/ob and lean control mice (b), and HFD and chow-diet-fed mice (c) (n = 4). d–l, Mice were immunized with ovalbumin/complete Freund’s adjuvant. Lymphocytes in draining lymph nodes were analysed for cytokine expression on day 7 (n = 4). Flow cytometry analysis of IL-22+ lymphocytes (d–f), percentage of IL-22+ cells (g–i), IL-17A+ cells (j, k), and IFN-γ+ cells (l) within the CD4+ population in db/db and lean controls (d, g, j, l), ob/ob and lean controls (e, h, k), and HFD and chow-diet-fed mice (f, i). The number at the top right-hand corner of each FACS plot is the percentage of IL-22+ cells within CD4+ cells (d–f). m, Percentage of Foxp3+ cells within CD4+ T cells from spleens of naive WT, db/db, and ob/ob mice (n = 5). Error bars, s.e.m. NS, not significant. *P < 0.05, **P < 0.01, ***P < 0.001, unpaired Student’s t-test. Data shown are representative of three (d, e, g, h, j–l) or two (a–c, f, i, m) independent experiments.
Extended Data Figure 2 Susceptibility of ob/ob and DIO mice to C. rodentium infection.
a, b, Body weight (a) and survival (b) of ob/ob and lean control mice (n = 10) infected with C. rodentium. c, d, Colon histology of lean control (c) or ob/ob (d) mice on day 8 after infection. Arrows, bacterial colonies; vertical bar, submucosal oedema; horizontal bar, 200 μm. e, Clinical score determined by colon histology (n = 5). f, g, Bacterial burden in liver (f) and spleen (g) on day 7 (n = 5). h, Survival of mice fed with HFD or chow diet infected with C. rodentium (n = 10). i, Bacterial burden in colon on day 18 (n = 5). Error bars, s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, Log rank test (b) or unpaired Student’s t-test (e–g, i). Data shown are representative of two independent experiments.
Extended Data Figure 3 Reduced IL-23 induction contributes to the compromised IL-22 expression and host defence during C. rodentium infection in obese mice.
a–c, WT, db/db, and ob/ob mice on day 4 after infection. a, Percentage of ILCs, IL-22+ ILCs, and IL-22+ CD4+ T cells within leukocytes in colons after ex vivo stimulation (n = 3). b, c, Relative Il23a (b) and Il12b (c) mRNA expression in colons (n = 5). d, e, WT and ob/ob mice were infected with C. rodentium and the hydrodynamic tail vein injected with either a control or IL-23-encoding plasmid. d, Relative Il22 mRNA expression on day 4 (n = 10 for WT, n = 6 for ob/ob + Ctrl and ob/ob + IL-23). e, Survival (n = 10 for WT and ob/ob + Ctrl, n = 8 for ob/ob + IL-23). f–h, db/db mice were infected with C. rodentium and treated with control IgG or IL-22-Fc (n = 5 for uninfected and IL-22-Fc, n = 4 for control). f, Colon histology on day 6. Arrows, bacterial colonies; horizontal bar, 100 μm. g, Clinical score determined by colon histology. h, Bacterial burden in spleen (left) and liver (right) on day 8. Error bars, s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, unpaired Student’s t-test (b–d, g, h) or log rank test (e). Data shown are representative of two independent experiments.
Extended Data Figure 4 Role of IL-22 pathway in metabolic disorders.
a, Strategy for generation of IL-22R1 KO mice. b, Il22ra1 mRNA expression in colons from IL-22R1 KO and WT littermates (n = 5). c, Reg3b mRNA expression in colons from IL-22R1 KO and WT littermates 2 days after an injection with IL-22-Fc or control IgG (n = 5). d, e, IL-22R1 KO, IL-22 KO (n = 13), and WT littermate (n = 8) male mice were fed with HFD as in Fig. 2a–c. d, ITT of IL-22R1 KO mice as in Fig. 2c. e, GTT of IL-22 KO mice at 3 months with HFD. f–j, Eight-week-old C57BL/6 mice were concurrently fed with HFD and treated with IL-22-Fc or control IgG (n = 7). f, Body weight. g, Blood glucose. h, GTT on day 23. i, Fasted blood glucose on day 23. j, Epididymal fat-pad mass on day 25. Error bars, s.e.m.; NS, not significant. *P < 0.05, **P < 0.01, ***P < 0.001, unpaired Student’s t-test (b, c, i, j) or two-way ANOVA (d–h). Data shown are representative of three independent experiments.
Extended Data Figure 5 IL-22 ameliorates metabolic disorders in diabetic db/db mice.
db/db mice were treated with IL-22-Fc or control IgG for 3 weeks (n = 7). a, Abdominal photograph of representative mice. b, Body weight. c, Parametrial fat-pad mass. d, Blood glucose. e, Fed and fasted blood glucose. f, GTT. g, ITT. h, Normalized ITT. Error bars, s.e.m.; NS, not significant. *P < 0.05, **P < 0.01, ***P < 0.001, two-way ANOVA (b, d, f–h) or unpaired Student’s t-test (c, e). Data shown are representative of five independent experiments.
Extended Data Figure 6 IL-22 restores insulin homeostasis in db/db mice.
a–c, db/db mice were treated with IL-22-Fc or control IgG for 3 weeks. a, b, Serum insulin of fed (a) and 16 h fasted (b) mice. c, Insulin secretion in response to glucose injection after fasting for 16 h (n = 8). d–j, Pancreases were harvested and stained for insulin and glucagon (n = 7). d, e, Immunohistochemistry of pancreas islet from db/db mice treated with control IgG (d) or IL-22-Fc (e). Green, glucagon; red, insulin. The area surrounded by the red line shows islet area. Scale bar, 50 μm. f, Percentage of islet area within the total pancreas area. g, Percentage of β-cell area within the total islet area. h, Percentage of α-cell area within the total islet area. i, Average insulin staining intensity. j, Average glucagon staining intensity. Error bars, s.e.m.; NS, not significant. *P < 0.05, **P < 0.01, ***P < 0.001, unpaired Student’s t-test. Data shown are representative of two independent experiments.
Extended Data Figure 7 Role of IL-22-Fc in modulation of intestinal microbiota and chronic inflammation.
a–c, DIO mice were treated with IL-22-Fc or control IgG for 6 weeks (n = 8). Faecal DNA was analysed by real-time PCR. a, Relative levels of Bacteroidetes 16S rRNA. b, Relative levels of Firmicutes 16S rRNA. c, Ratio of Bacteroidetes to Firmicutes. d, e, DIO mice were treated with IL-22-Fc or control IgG for 10 weeks (n = 5). One group of IL-22-Fc-treated mice were co-housed with control treated mice (n = 5). d, Body weight. e, GTT. f, g, db/db mice were treated with IL-22-Fc or control IgG for 2 weeks (n = 10). f, Serum lipopolysaccharide. g, Real-time PCR analyses of Tnfa, Il1b, and Il6 mRNA expression in WAT. Error bars, s.e.m.; NS, not significant. *P < 0.05, **P < 0.01, ***P < 0.001, unpaired Student’s t-test (a, c, f, g) or two-way ANOVA (d, e). Data shown are representative of two independent experiments.
Extended Data Figure 8 The regulation of glucose homeostasis by IL-22-Fc is independent of its function in limiting food intake.
a, Accumulative food intake in the first week of the db/db mice in Extended Data Fig. 5. b, Serum PYY in db/db mice treated with IL-22-Fc or control IgG (n = 5). c, Four-hour food intake of IL-22-Fc or control IgG-dosed C57BL/6 male mice in a fast and re-fed study with a single injection of NPY2 R inhibitor, BIIE0246, or PBS (n = 5). d–f, Two groups of db/db mice (n = 6) were fed with food ad libitum and treated with IL-22-Fc or control IgG for 4 weeks. One group of db/db mice (n = 6) was fed with restricted food that matched the food intake of IL-22-Fc treated group, and treated with control IgG. d, Accumulative food intake of the first 2 weeks. e, Blood glucose. f, GTT. Error bars, s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, two-way ANOVA (a, d–f) or unpaired Student’s t-test (b, c). Data shown are representative of two independent experiments.
Extended Data Figure 9 IL-22-Fc, but not IL-20-Fc or IL-24-Fc, improves metabolic syndrome.
a, Stat3 phosphorylation in liver from WT mice treated with Fc proteins (200 μg) for 2 h in vivo. b, c, Liver enzymes of DIO mice (b) and db/db mice (c) treated with IL-22-Fc or control IgG for 4 weeks (n = 5). d, e, db/db mice were treated with IL-22-Fc (n = 9) or control IgG (n = 6) for 4 weeks. d, Hepatic triglyceride. e, WAT triglyceride. f, g, Il20rb (f) and Il10rb (g) mRNA expression in indicated tissues of WT mice. h, i, db/db mice were treated with Fc proteins for 2 weeks (n = 10). h, Blood glucose. i, GTT. Error bars, s.e.m. *P < 0.05, ***P < 0.001, unpaired Student’s t-test (b–e) or two-way ANOVA (h, i).
Rights and permissions
About this article
Cite this article
Wang, X., Ota, N., Manzanillo, P. et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 514, 237–241 (2014). https://doi.org/10.1038/nature13564
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13564
This article is cited by
-
Effects of probiotic administration on overweight or obese children: a meta-analysis and systematic review
Journal of Translational Medicine (2023)
-
Opportunities and challenges: interleukin-22 comprehensively regulates polycystic ovary syndrome from metabolic and immune aspects
Journal of Ovarian Research (2023)
-
Dimethyl itaconate ameliorates cognitive impairment induced by a high-fat diet via the gut-brain axis in mice
Microbiome (2023)
-
Supplementation with the Probiotic Strains Bifidobacterium longum and Lactiplantibacillus rhamnosus Alleviates Glucose Intolerance by Restoring the IL-22 Response and Pancreatic Beta Cell Dysfunction in Type 2 Diabetic Mice
Probiotics and Antimicrobial Proteins (2023)
-
Discrete interplay of gut microbiota L-tryptophan metabolites in host biology and disease
Molecular and Cellular Biochemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.