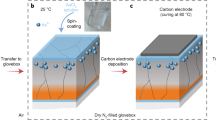
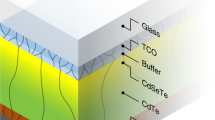
Abstract
Cadmium telluride, CdTe, is now firmly established as the basis for the market-leading thin-film solar-cell technology. With laboratory efficiencies approaching 20 per cent1, the research and development targets for CdTe are to reduce the cost of power generation further to less than half a US dollar per watt (ref. 2) and to minimize the environmental impact. A central part of the manufacturing process involves doping the polycrystalline thin-film CdTe with CdCl2. This acts to form the photovoltaic junction at the CdTe/CdS interface3,4 and to passivate the grain boundaries5, making it essential in achieving high device efficiencies. However, although such doping has been almost ubiquitous since the development of this processing route over 25 years ago6, CdCl2 has two severe disadvantages; it is both expensive (about 30 cents per gram) and a water-soluble source of toxic cadmium ions, presenting a risk to both operators and the environment during manufacture. Here we demonstrate that solar cells prepared using MgCl2, which is non-toxic and costs less than a cent per gram, have efficiencies (around 13%) identical to those of a CdCl2-processed control group. They have similar hole densities in the active layer (9 × 1014 cm−3) and comparable impurity profiles for Cl and O, these elements being important p-type dopants for CdTe thin films. Contrary to expectation, CdCl2-processed and MgCl2-processed solar cells contain similar concentrations of Mg; this is because of Mg out-diffusion from the soda-lime glass substrates and is not disadvantageous to device performance. However, treatment with other low-cost chlorides such as NaCl, KCl and MnCl2 leads to the introduction of electrically active impurities that do compromise device performance. Our results demonstrate that CdCl2 may simply be replaced directly with MgCl2 in the existing fabrication process, thus both minimizing the environmental risk and reducing the cost of CdTe solar-cell production.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Green, M. A., Emery, K., Hishikawa, Y., Warta, W. & Dunlop, E. D. Solar cell efficiency tables (version 43). Prog. Photovolt. Res. Appl. 22, 1–9 (2014)
Zweibel, K. The impact of tellurium supply on cadmium telluride photovoltaics. Science 328, 699–701 (2010)
McCandless, B. E., Moulton, L. V. & Birkmire, R. W. Recrystallization and sulfur diffusion in CdCl2-treated CdTe/CdS thin films. Prog. Photovolt. Res. Appl. 5, 249–260 (1997)
Moutinho, H. R. et al. Studies of recrystallization of CdTe thin films after CdCl2 treatment. In Conference Record of the Twenty Sixth IEEE Photovoltaic Specialists Conference (Anaheim, California) 431–434 (IEEE, 1997)
Edwards, P. R., Galloway, S. A. & Durose, K. EBIC and luminescence mapping of CdTe/CdS solar cells. Thin Solid Films 372, 284–291 (2000)
Basol, B. M., Ou, S. S. & Stafsudd, O. M. Type conversion, contacts, and surface effects in electroplated CdTe-films. J. Appl. Phys. 58, 3809–3813 (1985)
Fthenakis, V. M. & Kim, H. C. CdTe photovoltaics: life cycle environmental profile and comparisons. Thin Solid Films 515, 5961–5963 (2007)
Mazzamuto, S. et al. A study of the CdTe treatment with a freon gas such as CHF2Cl. Thin Solid Films 516, 7079–7083 (2008)
Balarew, C. Solubilities in seawater-type systems—some technical and environmental friendly applications. Pure Appl. Chem. 65, 213–218 (1993)
Liu, Z. S. & Chang, S. K. C. Optimal coagulant concentration, soymilk and tofu quality as affected by a short-term model storage of proto soybeans. J. Food Process. Preserv. 32, 39–59 (2008)
Demtsu, S. H. & Sites, J. R. Effect of back-contact barrier on thin-film CdTe solar cells. Thin Solid Films 510, 320–324 (2006)
Drayton, J., Geisthardt, R., Raguse, J. & Sites, J. R. Metal chloride passivation treatments for CdTe solar cells. Mater. Res. Soc. Symp. Proc. 1538, 269–274 (2013)
Waters, D. et al. Surface analysis of CdTe after various pre-contact treatments. In Proceedings of the 2nd World Conference and Exhibition of Photovoltaic Solar Energy Conversion 1031–1034 (European Commission Joint Research Centre, 1995)
Bätzner, D. L., Oszan, M. E., Bonnet, D. & Bucher, K. Device analysis methods for physical cell parameters of CdTe/Cds solar cells. Thin Solid Films 361/362, 288–292 (2000)
Fahrenbruch, A. L. Exploring back contact technology to increase CdS/CdTe solar cell efficiency. Mater. Res. Soc. Symp. Proc. 1012, abstr. 1012-Y07-05. (2007)
Kosyachenko, L. & Toyama, T. Current-voltage characteristics and quantum efficiency spectra of efficient thin-film CdS/CdTe solar cells. Sol. Energy Mater. Sol. Cells 120, 512–520 (2014)
Proskuryakov, Y. Y. et al. Doping levels, trap density of states and the performance of co-doped CdTe(As,Cl) photovoltaic devices. Sol. Energy Mater. Sol. Cells 93, 1572–1581 (2009)
Burgelman, M., Nollet, P. & Degrave, S. Electronic behaviour of thin-film CdTe solar cells. Appl. Phys. A 69, 149–153 (1999)
Li, J. V. et al. Theoretical analysis of effects of deep level, back contact, and absorber thickness on capacitance-voltage profiling of CdTe thin-film solar cells. Sol. Energy Mater. Sol. Cells 100, 126–131 (2012)
Tyan, Y. S., Vazan, F. & Barge, S. Effect of oxygen on thin-film CdS/CdTe solar cells. In Conference Record of the Twenty Sixth IEEE Photovoltaic Specialists Conference (Kissimmee, Florida) 840–845 (IEEE, 1984)
Wu, X. et al. Nanostructured CdS: O film: preparation, properties, and application. In 11th International Conference on II–VI Compounds Proceedings 1062–1066 (Wiley-VCH, 2004)
Barth, K. L., Enzenroth, R. A. & Sampath, W. S. Consistent processing and long term stability of CdTe devices. In Conference Record of the Twenty First IEEE Photovoltaic Specialists Conference (Lake Buena Vista, Florida) 323–326 (IEEE, 2005)
Major, J. D., Proskuryakov, Y. Y. & Durose, K. Impact of CdTe surface composition on doping and device performance in close space sublimation deposited CdTe solar cells. Prog. Photovolt. Res. Appl. 21, 436–443 (2013)
Blood, P. & Orton, J. The Electrical Characterisation of Semiconductors: Majority Carriers and Electron States 220–264 (Academic, 1992)
Kobyakov, P. S., Geisthardt, R., Cote, T. & Sampath, W. S. Growth and characterization of Cd1 −x Mg x Te thin films for possible application in high-efficiency solar cells. In Conference Record of the Twenty Sixth IEEE Photovoltaic Specialists Conference (Austin, Texas) 160–163 (IEEE, 2012)
Hsiao K-J. & Sites, J. R. Electron reflector to enhance photovoltaic efficiency: application to thin-film CdTe solar cells. Prog. Photovolt. Res. Appl. 20, 486–489 (2012)
Acknowledgements
We thank T. Veal for assistance in manuscript preparation and the Engineering and Physical Sciences Research Council for funding support.
Author information
Authors and Affiliations
Contributions
J.D.M. and R.E.T. conceived the experiments. J.D.M. fabricated and tested the solar-cell devices. L.J.P. performed C–V measurements. J.D.M. and K.D. discussed the results and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 J–V–T data for cells with various chloride treatments.
Current density versus voltage curves measured as a function of temperature (J–V–T) with inset back-contact barrier height values ϕb, determined for the highest-efficiency contacts for: the CdCl2-treated device (a), the MgCl2-vapour-treated device (b), the NaCl-treated device (c) and the high-efficiency cell (see Extended Data Fig. 3) treated with a 1 M MgCl2/H2O solution and addition of 2 nm Cu to the back contact (d). Values for the back-contact barrier height are extracted by fitting to the temperature dependence of the series resistance Rs (see Methods).
Extended Data Figure 2 Stability measurements for CdCl2- and MgCl2-treated cells.
J–V curves for devices treated with the MgCl2 vapour process (a) and the CdCl2 treatment (b). J–V curves were measured immediately after processing and then after 6 months of storage under ambient conditions. Performance degradation over the 6-month period was assessed from the average shift in efficiency, fill factor, short circuit current density Jsc and open circuit voltage Voc for nine contacts over this period. The averages for the ratio of initial and final performances, along with the associated error, are given for the MgCl2 vapour treatment (c) and the CdCl2 treatment (d).
Extended Data Figure 3 High-efficiency MgCl2-treated devices.
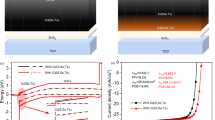
Performance of CdTe devices treated with MgCl2 is further improved following device optimization and the use of a 1 M MgCl2/H2O solution. A 2-nm Cu layer is added to the back contact, the ZnO buffer layer is replaced with a nanostructured CdS:O layer and CdS thickness is reduced to about 40 nm. a, J–V curves for the unimproved MgCl2-vapour-treated device (13.5%), and the improved cell treated with MgCl2/H2O solution (15.7%). b, EQE curves for the same devices, showing minimised CdS/ZnO cut-off at short wavelength (300–525 nm) by use of CdS:O layer. c, Extracted device performance parameters from J–V data.
Rights and permissions
About this article
Cite this article
Major, J., Treharne, R., Phillips, L. et al. A low-cost non-toxic post-growth activation step for CdTe solar cells. Nature 511, 334–337 (2014). https://doi.org/10.1038/nature13435
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13435
This article is cited by
-
Ultra-thin CdTe film properties enhancement via eco-friendly MgCl2-assisted thermal treatment
Journal of Optics (2024)
-
Annealing evolution to MgCl2 treated CdSe absorber layers for solar cells
Journal of Materials Science: Materials in Electronics (2023)
-
Application of locational CdCl2 treatment to CdS thin films grown on an ultra-thin MgZnO for CdTe solar cells
Applied Physics A (2023)
-
Activation of thermally evaporated CdSe films employing different halides: an evolution to ion size impact on grain growth
Journal of Materials Science (2022)
-
Anisotropic mechanical responses and plastic deformation mechanisms of cadmium telluride under indentations
Applied Physics A (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.