Abstract
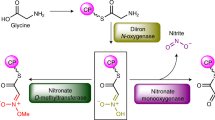
Sulphur is an essential element for life and is ubiquitous in living systems1,2. Yet how the sulphur atom is incorporated into many sulphur-containing secondary metabolites is poorly understood. For bond formation between carbon and sulphur in primary metabolites, the major ionic sulphur sources are the persulphide and thiocarboxylate groups on sulphur-carrier (donor) proteins3,4. Each group is post-translationally generated through the action of a specific activating enzyme. In all reported bacterial cases, the gene encoding the enzyme that catalyses the carbon–sulphur bond formation reaction and that encoding the cognate sulphur-carrier protein exist in the same gene cluster5. To study the production of the 2-thiosugar moiety in BE-7585A, an antibiotic from Amycolatopsis orientalis, we identified a putative 2-thioglucose synthase, BexX, whose protein sequence and mode of action seem similar to those of ThiG, the enzyme that catalyses thiazole formation in thiamine biosynthesis6,7. However, no gene encoding a sulphur-carrier protein could be located in the BE-7585A cluster. Subsequent genome sequencing uncovered a few genes encoding sulphur-carrier proteins that are probably involved in the biosynthesis of primary metabolites but only one activating enzyme gene in the A. orientalis genome. Further experiments showed that this activating enzyme can adenylate each of these sulphur-carrier proteins and probably also catalyses the subsequent thiolation, through its rhodanese domain. A proper combination of these sulphur-delivery systems is effective for BexX-catalysed 2-thioglucose production. The ability of BexX to selectively distinguish sulphur-carrier proteins is given a structural basis using X-ray crystallography. This study is, to our knowledge, the first complete characterization of thiosugar formation in nature and also demonstrates the receptor promiscuity of the A. orientalis sulphur-delivery system. Our results also show that co-opting the sulphur-delivery machinery of primary metabolism for the biosynthesis of sulphur-containing natural products is probably a general strategy found in nature.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
GenBank/EMBL/DDBJ
Protein Data Bank
Data deposits
The nucleotide sequences of the thiamine biosynthetic gene cluster, moeZ and the surrounding genes, the molybdopterin biosynthetic gene cluster, the cysteine biosynthetic gene cluster, and moaD2 and the surrounding genes have been deposited in the GenBank database under the accession numbers JN602207, JN602208, JN602209, JN602210 and JN602211, respectively. The atomic coordinates and structure factors for BexX-G6P and BexX–CysO have been deposited in the Protein Data Bank under the accession numbers 4N6Fand 4N6E, respectively.
References
Parry, R. J. in Comprehensive Natural Products Chemistry Vol. 1 (eds Meth-Cohn, O., Barton, D. & Nakanishi, K. ) 825–863 (Elsevier Science, 1999)
Fontecave, M., Ollagnier-de-Choudens, S. & Mulliez, E. Biological radical sulfur insertion reactions. Chem. Rev. 103, 2149–2166 (2003)
Mueller, E. G. Trafficking in persulfides: delivering sulfur in biosynthetic pathways. Nature Chem. Biol. 2, 185–194 (2006)
Kessler, D. Enzymatic activation of sulfur for incorporation into biomolecules in prokaryotes. FEMS Microbiol. Rev. 30, 825–840 (2006)
Burroughs, A. M., Iyer, L. M. & Aravind, L. Natural history of the E1-like superfamily: implication for adenylation, sulfur transfer, and ubiquitin conjugation. Proteins 75, 895–910 (2009)
Sasaki, E., Ogasawara, Y. & Liu, H.-w. A biosynthetic pathway for BE-7585A, a 2-thiosugar-containing angucycline-type natural product. J. Am. Chem. Soc. 132, 7405–7417 (2010)
Sasaki, E. & Liu, H.-w. Mechanistic studies of the biosynthesis of 2-thiosugar: evidence for the formation of an enzyme-bound 2-ketohexose intermediate in BexX-catalyzed reaction. J. Am. Chem. Soc. 132, 15544–15546 (2010)
Thibodeaux, C. J., Melançon, C. E. & Liu, H.-w. Unusual sugar biosynthesis and natural product glycodiversification. Nature 446, 1008–1016 (2007)
Thibodeaux, C. J., Melançon, C. E. & Liu, H.-w. Natural-product sugar biosynthesis and enzymatic glycodiversification. Angew. Chem. Int. Edn Engl. 47, 9814–9859 (2008)
Lin, C.-I., McCarty, R. M. & Liu, H.-w. The biosynthesis of nitrogen-, sulfur- and high-carbon chain-containing sugars. Chem. Soc. Rev. 42, 4377–4407 (2013)
Braunshausen, A. & Seebeck, F. P. Identification and characterization of the first ovothiol biosynthetic enzyme. J. Am. Chem. Soc. 133, 1757–1759 (2011)
Park, J. H. et al. Biosynthesis of the thiazole moiety of thiamin pyrophosphate (Vitamin B1). Biochemistry 42, 12430–12438 (2003)
Begley, T. P. Cofactor biosynthesis: an organic chemist’s treasure trove. Nat. Prod. Rep. 23, 15–25 (2006)
Jurgenson, C. T., Begley, T. P. & Ealick, S. E. The structural and biochemical foundations of thiamin biosynthesis. Annu. Rev. Biochem. 78, 569–603 (2009)
Iyer, L. M., Burroughs, A. M. & Aravind, L. The prokaryotic antecedents of the ubiquitin-signaling system and the early evolution of ubiquitin-like β-grasp domains. Genome Biol. 7, R60 (2006)
Mihara, H. & Esaki, N. Bacterial cysteine desulfurases: their function and mechanisms. Appl. Microbiol. Biotechnol. 60, 12–23 (2002)
Cipollone, R., Ascenzi, P. & Visca, P. Common themes and variations in the rhodanese superfamily. IUBMB Life 59, 51–59 (2007)
Schwarz, G., Mendel, R. R. & Ribbe, M. W. Molybdenum cofactors, enzymes and pathways. Nature 460, 839–847 (2009)
Burns, K. E. et al. Reconstitution of a new cysteine biosynthetic pathway in Mycobacterium tuberculosis. J. Am. Chem. Soc. 127, 11602–11603 (2005)
Jurgenson, C. T., Burns, K. E., Begley, T. P. & Ealick, S. E. Crystal structure of a sulfur carrier protein complex found in the cysteine biosynthetic pathway of Mycobacterium tuberculosis. Biochemistry 47, 10354–10364 (2008)
Rudolph, M. J., Wuebbens, M. M., Rajagopalan, K. V. & Schindelin, H. Crystal structure of molybdopterin synthase and its evolutionary relationship to ubiquitin activation. Nature Struct. Biol. 8, 42–46 (2001)
Settembre, E. C. et al. Thiamin biosynthesis in Bacillus subtilis: structure of the thiazole synthase/sulfur carrier protein complex. Biochemistry 43, 11647–11657 (2004)
Shigi, N., Sakaguchi, Y., Asai, S., Suzuki, T. & Watanabe, K. Common thiolation mechanism in the biosynthesis of tRNA thiouridine and sulphur-containing cofactors. EMBO J. 27, 3267–3278 (2008)
Voss, M., Nimtz, M. & Leimkühler, S. Elucidation of the dual role of mycobacterial MoeZR in molybdenum cofactor biosynthesis and cysteine biosynthesis. PLoS ONE 6, e28170 (2011)
Delcher, A. L., Bratke, K. A., Powers, E. C. & Salzberg, S. L. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 23, 673–679 (2007)
Lowe, T. M. & Eddy, S. R. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25, 955–964 (1997)
Lagesen, K. et al. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 35, 3100–3108 (2007)
Kinsland, C., Taylor, S. V., Kelleher, N. L., McLafferty, F. W. & Begley, T. P. Overexpression of recombinant proteins with a C-terminal thiocarboxylate: implications for protein semisynthesis and thiamin biosynthesis. Protein Science 7, 1839–1842 (1998)
Bradford, M. M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976)
Sörbo, B. Enzymic transfer of sulfur from mercatopyruvate to sulfate or sulfinates. Biochim. Biophys. Acta 24, 324–329 (1957)
Otwinowski, Z. M. W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007)
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010)
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010)
Murshudov, G. N. et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D 67, 355–367 (2011)
Geoghegan, K. F. et al. Spontaneous α-N-6-phosphogluconoylation of a ‘His tag’ in Escherichia coli: the cause of extra mass of 258 or 178 Da in fusion proteins. Anal. Biochem. 267, 169–184 (1999)
Di Tommaso, P. et al. T-Coffee: a web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Res. 39, W13–W17 (2011)
Corpet, F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16, 10881–10890 (1988)
Gouet, P., Robert, X. & Courcelle, E. ESPript/ENDscript: extracting and rendering sequence and 3D information from atomic structures of proteins. Nucleic Acids Res. 31, 3320–3323 (2003)
Schlesinger, P. & Westley, J. An expanded mechanism for rhodanese catalysis. J. Biol. Chem. 249, 780–788 (1974)
Chowdhury, M. M., Dosche, C., Löhmannsröben, H. & Leimkühler, S. Dual role of the molybdenum cofactor biosynthesis protein MOCS3 in tRNA thiolation and molybdenum cofactor biosynthesis in humans. J. Biol. Chem. 287, 17297–17307 (2012)
Matthies, A., Rajagopalan, K. V., Mendel, R. R. & Leimkühler, S. Evidence for the physiological role of a rhodanese-like protein for the biosynthesis of the molybdenum cofactor in humans. Proc. Natl Acad. Sci. USA 101, 5946–5951 (2004)
Marelja, Z., Stöcklein, W., Nimtz, M. & Leimkühler, S. A novel role for human Nfs1 in the cytoplasm: Nfs1 acts as a sulfur donor for MOCS3, a protein involved in molybdenum cofactor biosynthesis. J. Biol. Chem. 283, 25178–25185 (2008)
Beinert, H. Semi-micro methods for analysis of labile sulfide and of labile sulfide plus sulfane sulfur in unusually stable iron–sulfur proteins. Anal. Biochem. 131, 373–378 (1983)
Acknowledgements
We thank C.-H. Wong and R. Chen, as well as Academia Sinica for funding, for the whole-genome draft sequencing; and the staff at the Analytical Instrumentation Facility of the College of Pharmacy (University of Texas), the Advanced Photon Source (APS) Northeastern Collaborative Access Team (NE-CAT) beamlines and the Cornell High Energy Synchrotron Source (CHESS) beamlines (Cornell University) for their assistance in data collection. We also thank D. Kim for assistance with the early biochemical experiments, C. Kinsland for providing the cysO/pTYB1 clone, Y. Zhang for help with the structure determination of BexX–CysO and L. Kinsland for help in preparing the manuscript. This work was supported in part by grants from the National Institutes of Health (NIH) (GM035906 to H.-W.L. and DK67081 to S.E.E.) and the Welch Foundation (F-1511 to H.-W.L.). The X-ray crystallography work was conducted at the APS NE-CAT beamlines, which are supported by award GM103403 from the National Institute of General Medical Sciences, NIH. Use of the APS is supported by the US Department of Energy, Office of Basic Energy Sciences, under contract no. DE-AC02-06CH11357. The use of the CHESS is supported by the National Science Foundation (DMR-0936384) and the National Institute of General Medical Sciences, NIH (GM103485).
Author information
Authors and Affiliations
Contributions
H.-W.L. provided the scientific direction and the overall experimental design for the studies. E.S. and H.G.S. designed and performed the biochemical experiments. X.Z. and S.E.E. were responsible for the crystal structure studies. M.-Y.J.L., T.-L.L., A.O., J.-Y.L. and Y.-H.C. carried out the whole-genome sequencing and gene annotation. E.S., X.Z., H.G.S., S.E.E. and H.-W.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Structures of BexX and CysO from Amycolatopsis orientalis.
a, A stereo ribbon diagram of the (βα)8-barrel fold of BexX is shown from the top view. The α-helices, β-strands and loops are marked in blue, green and yellow, respectively. The ketone-intermediate (2) formed by Lys 110 and G6P is shown as sticks and coloured in purple. b, The typical secondary structure composition of the classical (βα)8-barrel is shown as a topology model; the conserved Lys 110 is highlighted in red. c, The quaternary structure of BexX is shown as a ribbon diagram with two monomers coloured by chain. d, A ribbon diagram of CysO from the BexX–CysO structure. Secondary structural elements are coloured blue for α-helices, green for β-strands and yellow for loops. e, A topology diagram of CysO. f, Data collection and refinement statistics. One crystal was used for each of the two data sets. *The values in parentheses are for the highest-resolution shell.
Extended Data Figure 2 Putative thiamine, molybdenum cofactor and cysteine biosynthetic genes found in A. orientalis and their proposed functions.
a, Organization of the putative thiamine biosynthetic gene cluster and the proposed thiamine biosynthetic pathway in A. orientalis. *The genes encoding MoeZ and ThiL (one of the genes involved in thiazole biosynthesis) are not found in the gene cluster. The gene encoding the ThiS-activating enzyme, ThiF, is also absent from the genome. †Two genes encoding proteins homologous to ThiD are found in the gene cluster. b, Organization of the putative molybdopterin biosynthetic gene cluster and the proposed molybdenum cofactor biosynthetic pathway in A. orientalis. *The genes encoding MoeZ and MoeA are not found in the gene cluster. The gene encoding the MoaD-activating enzyme, MoeB, is also absent from the genome. c, Organization of the putative cysteine biosynthetic gene cluster and the proposed cysteine biosynthetic pathway in A. orientalis. *The gene encoding MoeZ is not found in the gene cluster. d, Organization near the moaD homologue, moaD2, found in the A. orientalis genome. e, Organization near moeZ in the A. orientalis genome and the conserved domains of MoeZ predicted by BLAST analysis.
Extended Data Figure 3 ESI–MS analyses of the MoeZ-catalysed activation of sulphur-carrier proteins and SDS–PAGE separation of the purified proteins.
a, Reaction scheme of the MoeZ-catalysed activation of ThiS. b–e, Deconvoluted ESI–MS analyses of as-isolated ThiS (b), ThiS in the presence of MoeZ and ATP (c), ThiS in the presence of MoeZ, ATP and bisulphide (d) and ThiS in the presence of bisulphide (control) (e). The calculated molecular masses are shown as the neutral form in the upper right corner. Analysis of purified N-His6-ThiS (where N denotes amino terminal) shows two mass signals (observed (obsd), 8,646 and 8,824 Da) consistent with the calculated molecular mass of the recombinant enzyme in its native and N-gluconoylated form (where N denotes amino terminal) (calcd, 8,647 and 8,825 Da). Gluconoylation of the N-terminal His6 tag is a known post-translational modification when expressing recombinant proteins in E. coli37. Such a modification should not affect ThiS activity, because the predicted active site for ThiS is at the C terminus. Indeed, when N-His6-ThiS was incubated with N-His6-MoeZ and ATP, a mass spectrometric signal corresponding to adenylated N-His6-ThiS (9) was detected together with a few peaks that were probably derived from a reaction of the labile adenylated ThiS with buffer components (see c). f–k, Deconvoluted ESI–MS analyses of as-isolated MoaD (N-His6-MoaD, 105 amino acids; calcd, 11,022 Da) (f), as-isolated CysO (N-His6-CysO, 109 amino acids, and its N-gluconoylated derivative; calcd, 11,688 and 11,866 Da, respectively) (g), as-isolated MoaD2 (N-His6-MoaD2, 115 amino acids, and its N-gluconoylated derivative; calcd, 12,473 and 12,651 Da, respectively) (h), MoaD incubated with MoeZ, ATP and NaSH (N-His6-MoaD-COSH; calcd, 11,038 Da) (i), CysO incubated with MoeZ, ATP and NaSH (N-His6-CysO-COSH and its N-gluconoylated derivative; calcd, 11,704 and 11,882 Da, respectively) (j) and MoaD2 incubated with MoeZ, ATP and NaSH (N-His6-MoaD-COSH and its N-gluconoylated derivative; calcd, 12,489 and 12,667 Da, respectively) (k). l, SDS–PAGE gel of purified sulphur-carrier proteins, MoeZ and CD4: N-His6-ThiS (85 amino acids, 8.7 kDa, lane 2), N-His6-MoaD2 (115 amino acids, 12.5 kDa, lane 3), N-His6-CysO (109 amino acids, 11.7 kDa, lane 4), N-His6-MoeZ (421 amino acids, 45.0 kDa, lane 5), N-His6-MoaD (105 amino acids, 11.0 kDa, lane 7) and N-His6-CD4 (417 amino acids, 43.3 kDa, lane 9). The molecular weight markers are 220, 160, 120, 100, 90, 80, 70, 60, 50, 40, 30, 25, 20, 15 and 10 kDa (top to bottom, lanes 1, 6 and 8). The protein MoaD did not express well, and the partially purified protein solution contained significant amounts of endogenous proteins from the E. coli host.
Extended Data Figure 4 BexX-catalysed 2-thio-d-glucose-6-phosphate formation followed by alkaline phosphatase treatment.
a, Reaction scheme to synthesize the expected bimane derivative. b, HPLC traces of the C-His6-BexX-catalysed reactions (where C denotes carboxy terminal) using N-His6-ThiS, N-His6-CysO or N-His6-MoaD2, and the control reactions. The thiosugar product was treated with alkaline phosphatase (CIP) and then derivatized with mBBr. HPLC analysis of the synthetic standard of 2-thio-d-glucose-bimane is shown in the bottom trace (trace 7). c, High-resolution ESI–MS (positive) of the isolated product peak (2-thio-d-glucose-bimane C16H22N2NaO7S+ [M + Na]+, calcd, 409.1040 Da; obsd, 409.1038 Da).
Extended Data Figure 5 Sequence alignment of A. orientalis CysO, MoaD2 and ThiS and hydrophobic interactions for BexX complexes.
a, Sequence alignment was based on structural supersession using the programs 3D-Coffee38, MultAlin39 and ESPript40. The main differences between CysO (or MoaD2) and ThiS result from an insertion of ten residues between β1 and β2 of CysO (or MoaD2) and an insertion of 14 (or 15) residues between α1 and β3 of CysO (or MoaD2). The first insertion includes the short helix 3101, and the second includes helix α2. Both of these insertions are involved in the BexX–CysO (or BexX–MoaD2) interface. Ten interface residues (red stars and red triangles) are conserved between CysO and MoaD2; however, only four of these residues are conserved in ThiS (red triangles). Two differences between CysO and MoaD2 represent conservative substitutions; while Thr 9 and Ala 86 in CysO are replaced by Gly 11 and Ser 92 in MoaD2, the interface interaction is contributed by hydrogen bonds that are formed by the backbone atoms. b–d, Hydrophobic interactions of BexX–CysO (b), BexX–MoaD2 (c) and BexX–ThiS (d). BexX monomers are shown as grey ribbon diagrams with hydrophobic interaction regions coloured in cyan. CysO, MoaD2 and ThiS are shown as cartoons and coloured in green, blue and yellow, respectively. Hydrophobic interaction regions in sulphur-carrier proteins are coloured in red. The α-helices and β-strands in BexX and the sulphur-carrier proteins are labelled in black and red, respectively.
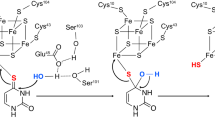
Extended Data Figure 6 The A. orientalis BexX–CysO interface, predicted hydrogen bonds between BexX with sulphur-carrier proteins, and a comparison of the BexX–CysO interface with the Bacillus subtilis ThiG–ThiS interface.
a, Interacting surfaces of BexX (left) and CysO (right). The surface is colour coded by atom type (oxygen, red; nitrogen, blue; carbon, green). Non-interacting surfaces are shown in grey. b, Hydrogen bonds on the surface of BexX with CysO are shown as black dashes. c, Hydrogen bonds formed by the C-terminal tail of CysO and the surrounding residues from BexX are shown as black dashes. The Fo − Fc simulated annealing omit map of the C-terminal residues (Ala-Val-Ala-Gly-Gly) is rendered in grey and contoured at 3.0σ. Residues are shown as sticks with the carbon atoms in grey for BexX and green for CysO. CysO residues are labelled in red; BexX residues are labelled in black. d, Predicted hydrogen bonds between BexX and other sulphur-carrier proteins. The hydrogen-bonding scheme for the BexX–CysO complex (9 of 12 involve the C-terminal tail) is conserved in the model of the BexX–MoaD2 complex. e, The interface between BexX (blue) and CysO (pink). Secondary structural elements of CysO are labelled in black, the β2 and α2 elements in BexX are labelled in red. f, The interface between ThiG (grey) and ThiS (yellow) from B. subtilis. Secondary structural elements of ThiS are labelled in black, and the β2 and α2 elements in ThiG are labelled in red. The β2–α2 loop region in BexX and ThiG is highlighted in red. For CysO, 3101 and α2 form hydrophobic contacts with the β2–α2 loop and α2 of BexX. ThiG also uses its β2–α2 loop to interact with ThiS; however, ThiS uses two different loop regions to form the interface. In addition, the β2–α2 loop of BexX is closer to the (βα)8-barrel than in ThiG, in which the β2–α2 loop extends outwards and covers the top of ThiS.
Extended Data Figure 7 MoeZ-dependent protein thiocarboxylate formation in sulphur-carrier proteins using thiosulphate as the sulphur source.
a–d, Deconvoluted ESI–MS of MoaD2 incubated with MoeZ (the observed peaks are consistent with the calculated molecular masses of N-His6-MoaD2-COSH (12,489 Da), N-His6-MoaD2-glycerol (12,547 Da), and N-gluconoylated-His6-MoaD2-COSH (12,667 Da)) (a), MoaD2 incubated with the MoeZ(Cys360Ala) mutant (the observed peaks are consistent with the calculated molecular masses of N-His6-MoaD2 (12,473 Da) and N-His6-MoaD2-glycerol (12,547 Da)) (b), CysO incubated with MoeZ (the observed peaks are consistent with the calculated molecular masses of N-His6-CysO-COSH (11,704 Da), N-His6-CysO-glycerol (11,762 Da) and N-gluconoylated-His6-MoaD2-COSH (11,882 Da)) (c), and CysO incubated with the MoeZ(Cys360Ala) mutant (the observed peaks are consistent with the calculated molecular masses of N-His6-CysO (11,688 Da), N-His6-CysO-glycerol (11,762 Da), their N-gluconoylated derivatives (11,866 Da, and 11,940 Da, respectively) and N-His6-CysO-AMP (12,017 Da)) (d). Observed masses corresponding to protein thiocarboxylate are shown in red. Two peaks corresponding to the dehydration of N-His6-MoaD2 and N-His6-CysO were probably caused by in-source collision-induced dissociation (CID) during the ESI–MS analysis. e, Kinetic parameters for the thiosulphate:cyanide sulphur transferase activity of MoeZ from A. orientalis. Bovine liver rhodanese is a typical rhodanese enzyme. Compared with bovine rhodanese, human molybdopterin synthase sulphurase (human MOCS3) displayed much lower thiosulphate:cyanide sulphur transferase activity41,42. In the case of human MOCS3, l-cysteine and cysteine desulphurase are proposed as the physiological sulphur source over thiosulphate because of its lower rhodanese activity43,44. However, this may not be the case for MoeZ from A. orientalis because its rhodanese activity is comparable to bovine liver rhodanese.
Extended Data Figure 8 Relative adenylation activity of MoeZ and the MoeZ(Cys360Ala) mutant.
a, Reaction scheme for the MoeZ-catalysed adenylation activity assay. The adenylation activities of MoeZ and its Cys360Ala mutant were inferred using a colorimetric assay to monitor the production of AMP (indicated by a decrease in NADH at 340 nm) when MoeZ or its Cys360Ala mutant was co-incubated with a sulphur-carrier protein (MoaD2) in the presence of ATP, NaSH, adenylate kinase (AK), pyruvate kinase (PK) and lactate dehydrogenase (LDH). b, The relative adenylation activity of MoeZ (open circles) and its Cys360Ala mutant (filled circles), as well as a no MoeZ/MoeZ(Cys360Ala) control (open squares), was measured by the coupled enzyme assay, as described in a. Little difference in the decrease in absorption at 340 nm was observed between MoeZ and its Cys360Ala mutant (compared with the control with no MoeZ), suggesting that the mutation at Cys360 had little effect on the adenylation activity of MoeZ.
Extended Data Figure 9 BexX-catalysed 2-thiosugar formation using various sulphur sources.
a, b, Reaction scheme for C-His6-BexX-catalysed 2-thiosugar formation using N-His6-MoeZ, N-His6-MoaD2 and thiosulphate (a) or l-cysteine and the cysteine desulphurase (CD4) from A. orientalis (b). The reactions were carried out in the absence of reducing agent to avoid complications from the generation of bisulphide from protein persulphide (*see also below). Under these conditions, MoeZ cannot be regenerated after single turnover. The thiosugar product was derivatized with mBBr and then treated with alkaline phosphatase (CIP) to yield 2-thio-d-glucose-bimane (2SG-bimane). c, d, The 2SG-bimane product concentrations at different time points of incubation with thiosulphate (c) or l-cysteine and CD4 (d) as the sulphur source were estimated on the basis of the product peak area of each HPLC trace. The 2SG-bimane synthetic standard (10, 25, 50, 77, 100 and 200 μM) was used for calibration. The filled and open circles denote product formation from the incubation with N-His6-MoeZ and the N-His6-MoeZ(Cys360Ala) mutant, respectively. *The observed minor product formation with the MoeZ(Cys360Ala) mutant, l-cysteine and CD4 (see d, open circles) is probably caused by the formation of bisulphide, which could be generated on reduction of CD4-persulphide in the presence of free cysteine molecules. In fact, a small amount of bisulphide was detected under similar conditions with l-cysteine and CD4 (in the absence of other proteins and reducing agents) by the methylene blue assay within 15 min of incubation45.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary Table 1 and a Supplementary Discussion. (PDF 558 kb)
Rights and permissions
About this article
Cite this article
Sasaki, E., Zhang, X., Sun, H. et al. Co-opting sulphur-carrier proteins from primary metabolic pathways for 2-thiosugar biosynthesis. Nature 510, 427–431 (2014). https://doi.org/10.1038/nature13256
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13256
This article is cited by
-
Enzymatic synthesis of organoselenium compounds via C‒Se bond formation mediated by sulfur carrier proteins
Nature Synthesis (2024)
-
Thiocysteine lyases as polyketide synthase domains installing hydropersulfide into natural products and a hydropersulfide methyltransferase
Nature Communications (2021)
-
Biosynthesis of thiocarboxylic acid-containing natural products
Nature Communications (2018)
-
A bioinspired and biocompatible ortho-sulfiliminyl phenol synthesis
Nature Communications (2017)
-
New insights into paulomycin biosynthesis pathway in Streptomyces albus J1074 and generation of novel derivatives by combinatorial biosynthesis
Microbial Cell Factories (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.