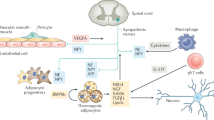
Abstract
Our understanding of adipose tissue biology has progressed rapidly since the turn of the century. White adipose tissue has emerged as a key determinant of healthy metabolism and metabolic dysfunction. This realization is paralleled only by the confirmation that adult humans have heat-dissipating brown adipose tissue, an important contributor to energy balance and a possible therapeutic target for the treatment of metabolic disease. We propose that the development of successful strategies to target brown and white adipose tissues will depend on investigations that elucidate their developmental origins and cell-type-specific functional regulators.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lelliott, C. & Vidal-Puig, A. J. Lipotoxicity, an imbalance between lipogenesis de novo and fatty acid oxidation. Int. J. Obes. Relat. Metab. Disord. 28, S22–S28 (2004).
Scherer, P. E. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 55, 1537–1545 (2006).
Hany, T. F. et al. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur. J. Nucl. Med. Mol. Imaging 29, 1393–1398 (2002).
Nedergaard, J. et al. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 293, E444–E452 (2007).
Virtanen, K. A. et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518–1525 (2009).
van Marken Lichtenbelt, W. D. et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508 (2009).
Cypess, A. M. et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 (2009). Refs 5–7 confirmed the presence of metabolically active BAT in adult humans.
Saito, M. et al. High incidence of metabolically active brown adipose effects of cold exposure and adiposity. Diabetes 58, 1526–1531 (2009).
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004).
Cannon, B. & Nedergaard, J. Thermogenesis challenges the adipostat hypothesis for body-weight control. Proc. Nutr. Soc. 68, 401–407 (2009).
Feldmann, H. M., Golozoubova, V., Cannon, B. & Nedergaard, J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 9, 203–209 (2009).
Lowell, B. B. et al. Development of obesity in transgenic mice after genetic ablation of adipose tissue. Nature 366, 740–742 (1993).
Bartelt, A. et al. Brown adipose tissue activity controls triglyceride clearance. Nature Med. 17, 200–205 (2011).
Arbeeny, C. M., Meyers, D. S., Hillyer, D. E. & Bergquist, K. E. Metabolic alterations associated with the antidiabetic effect of β 3-adrenergic receptor agonists in obese mice. Am. J. Physiol. 268, E678–E684 (1995).
Rothwell, N. J. & Stock, M. J. Luxuskonsumption, diet-induced thermogenesis and brown fat: the case in favour. Clin. Sci. 64, 19–23 (1983).
Loncar, D., Afzelius, B. A. & Cannon, B. Epididymal white adipose tissue after cold stress in rats. I. Nonmitochondrial changes. J. Ultrastruct. Mol. Struct. Res. 101, 109–122 (1988).
Young, P., Arch, J. R. & Ashwell, M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 167, 10–14 (1984).
Wu, J. et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012). This major study investigated the brown or beige phenotype of the adipocytes found in human supraclavicular BAT, and included a comprehensive gene expression analysis comparing rodent brown, beige and white adipocytes.
Waldén, T. B. et al. Recruited vs. nonrecruited molecular signatures of brown,“brite,” and white adipose tissues. Am. J. Physiol. Endocrinol. Metab. 302, E19–E31 (2012).
Sharp, L. Z. et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS ONE 7, e49452 (2012).
Petrovic, N. et al. Chronic peroxisome proliferator-activated receptor γ (PPAR-γ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocyt. J. Biol. Chem. 285, 7153–7164 (2010).
Shabalina, I. G. et al. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 5, 1196–1203 (2013).
Virtue, S. & Vidal-Puig, A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome–an allostatic perspective. Biochim. Biophys. Acta 1801, 338–349 (2010).
Rodeheffer, M. S., Birsoy, K. & Friedman, J. M. Identification of white adipocyte progenitor cells in vivo. Cell 135, 240–249 (2008).
Tang, W. et al. White fat progenitor cells reside in the adipose vasculature. Science 322, 583–586 (2008).
Carobbio, S., Rosen, B. & Vidal-Puig, A. Adipogenesis: new insights into brown adipose tissue differentiation. J. Mol. Endocrinol. 51, T75–T85 (2013).
Seale, P. et al. Pax7 is required for the specification of myogenic satellite cells. Cell 102, 777–786 (2000).
Lepper, C. & Fan, C. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis 48, 424–436 (2010)
Yin, H. et al. MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell Metab. 17, 210–224 (2013).
Seale, P. et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967 (2008). This study included a fate-mapping approach to identify the lineage origin of brown and white adipocytes, and identified PRDM16 as a 'cell-fate switch' that specifies a brown fat or muscle cell fate.
Timmons, J. A. et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc. Natl Acad. Sci. USA 104, 4401–4406 (2007).
Walden, T. B., Timmons, J. A., Keller, P., Nedergaard, J. & Cannon, B. Distinct expression of muscle-specific microRNAs (myomirs) in brown adipocytes. J. Cell. Physiol. 218, 444–449 (2009).
Sanchez-Gurmaches, J. et al. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab. 16, 348–362 (2012).
Shan, T. et al. Distinct populations of adipogenic and myogenic Myf5-lineage progenitors in white adipose tissues. J. Lipid Res. 54, 2214–2224 (2013).
Tran, K. V. et al. The vascular endothelium of the adipose tissue give rise to both white and brown fat cells. Cell Metab. 15, 222–229 (2012).
Gupta, R. K. et al. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial perivascular cells. Cell Metab. 15, 230–239 (2012).
Berry, R. & Rodeheffer, M. S. Characterization of the adipocyte cellular lineage in vivo. Nature Cell Biol. 15, 302–308 (2013).
Digby, J. E. et al. Thiazolidinedione exposure increases the expression of uncoupling protein 1 in cultured human preadipocytes. Diabetes 47, 138–141 (1998).
Petrovic, N., Shabalina, I. G., Timmons, J. A., Cannon, B. & Nedergaard, J. Thermogenically competent nonadrenergic recruitment in brown preadipocytes by a PPARg agonist. Am. J. Physiol. Endocrinol. Metab. 295, E287–E296 (2008).
Rosenwald, M., Perdikari, A., Rülicke, T. & Wolfrum, C. Bi-directional interconversion of brite and white adipocytes. Nature Cell Biol. 15, 659–667 (2013).
Wang, Q. A., Tao, C., Gupta, R. K. & Scherer, P. E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nature Med. 19, 1338–1344 (2013). Refs 40 and 41 both used genetically engineered mouse models to identify the adult origins of beige cells recruited by cold exposure.
Lee, Y.-H., Petkova, A. P., Mottillo, E. P. & Granneman, J. G. In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 15, 480–491 (2012).
Liu, W. et al. A heterogeneous lineage origin underlies the phenotypic and molecular differences of white and beige adipocytes. J. Cell Sci. 126, 3527–3532 (2013).
Hansen, J. B. & Kristiansen, K. Regulatory circuits controlling white versus brown adipocyte differentiation. Biochem. J. 398, 153–168 (2006).
Puigserver, P. Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-α. Int. J. Obes. 29, S5–S9 (2005).
Seale, P. et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 6, 38–54 (2007).
Seale, P. et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Invest. 121, 96–105 (2011).
Cohen, P. et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 156, 304–316 (2014).
Ohno, H., Shinoda, K., Ohyama, K., Sharp, L. Z. & Kajimura, S. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature 504, 163–167 (2013).
Frescas, D., Valenti, L. & Accili, D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J. Biol. Chem. 280, 20589–20595 (2005).
Rodgers, J. T. et al. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434, 113–118 (2005).
Lagouge, M. et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127, 1109–1122 (2006).
Fulco, M. et al. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol. Cell 12, 51–62 (2003).
Qiang, L. et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell 150, 620–632 (2012).
Trajkovski, M. & Lodish, H. MicroRNA networks regulate development of brown adipocytes. Trends Endocrinol. Metab. 24, 442–450 (2013).
Chen, Y. et al. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nature Commun. 4, 1769 (2013).
Sun, L. & Trajkovski, M. MiR-27 orchestrates the transcriptional regulation of brown adipogenesis. Metabolism 63, 272–282 (2014).
Mori, M., Nakagami, H., Rodriguez-Araujo, G., Nimura, K. & Kaneda, Y. Essential role for miR-196a in brown adipogenesis of white fat progenitor cells. PLoS Biol. 10, e1001314 (2012).
Sun, L. et al. MiR-193b-365, a brown fat enriched microRNA cluster, is essential for brown fat differentiation. Nature Cell Biol. 13, 958–965 (2011).
Trajkovski, M., Ahmed, K., Esau, C. C. & Stoffel, M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nature Cell Biol. 14, 1330–1335 (2012).
Liu, W. et al. miR-133a regulates adipocyte browning in vivo. PLoS Genet. 9, e1003626 (2013).
Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 26, 19–39 (2005).
Geerling, J. J. et al. Sympathetic nervous system control of triglyceride metabolism: novel concepts derived from recent studies. J. Lipid Res. 55, 180–189 (2014).
Collins, S. & Surwit, R. S. The β-adrenergic receptors and the control of adipose tissue metabolism and thermogenesis. Recent Prog. Horm. Res. 56, 309–328 (2001).
Schulz, T. J. & Tseng, Y.-H. Brown adipose tissue: development, metabolism and beyond. Biochem. J. 453, 167–178 (2013).
Schulz, T. J. et al. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature 495, 379–383 (2013).
Torp-Pedersen, C. et al. Cardiovascular responses to weight management and sibutramine in high-risk subjects: an analysis from the SCOUT trial. Eur. Heart J. 28, 2915–2923 (2007).
Arch, J. R. S. β3-adrenoceptor agonists: potential, pitfalls and progress. Eur. J. Pharmacol. 440, 99–107 (2002).
Cederberg, A. et al. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell 106, 563–573 (2001).
Grønning, L. M. et al. Reduced PDE4 expression and activity contributes to enhanced catecholamine-induced cAMP accumulation in adipocytes from FOXC2 transgenic mice. FEBS Lett. 580, 4126–4130 (2006).
Lidell, M. E. et al. The adipocyte-expressed forkhead transcription factor Foxc2 regulates metabolism through altered mitochondrial function. Diabetes 60, 427–435 (2011).
Whittle, A. J. et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 149, 871–885 (2012).
Tseng, Y.-H. et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454, 1000–1004 (2008).
Boon, M. R. et al. BMP7 activates brown adipose tissue and reduces diet-induced obesity only at subthermoneutrality. PLoS ONE 8, e74083 (2013).
Villarroya, F. & Vidal-Puig, A. Beyond the sympathetic tone: the new brown fat activators. Cell Metab. 17, 638–643 (2013).
Hondares, E. et al. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J. Biol. Chem. 286, 12983–12990 (2011).
Fisher, F. M. et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 26, 271–281 (2012).
Lafontan, M. et al. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol. Metab. 19, 130–137 (2008).
Bordicchia, M. et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Invest. 122, 1022–1036 (2012).
Moreno-Aliaga, M. J. et al. Cardiotrophin-1 is a key regulator of glucose and lipid metabolism. Cell Metab. 14, 242–253 (2011).
Boström, P. et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468 (2012).
Jones, S. A., Scheller, J. & Rose-John, S. Therapeutic strategies for the clinical blockade of IL-6 / gp130 signaling. J. Clin. Invest. 121, 3375–3383 (2011).
Roca-Rivada, A. et al. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 8, e60563 (2013).
Zhang, Y. et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 63, 514–525 (2014).
Wikstrom, J. D. et al. Hormone-induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO J. 33, 418–436 (2014).
Nguyen, K. D. et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480, 104–108 (2011).
Xue, Y. et al. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab. 9, 99–109 (2009).
Vitali, A. et al. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J. Lipid Res. 53, 619–629 (2012).
Xue, Y. et al. FOXC2 controls Ang-2 expression and modulates angiogenesis, vascular patterning, remodeling, and functions in adipose tissue. Proc. Natl Acad. Sci. USA 105, 10167–10172 (2008).
Elias, I. et al. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes 61, 1801–1813 (2012).
Sun, K. et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc. Natl Acad. Sci. USA 109, 5874–5879 (2012). The authors of this study dissected the contribution of angiogenesis to WAT function and dysfunction during the development of obesity compared with a state of existing obesity.
Trayhurn, P., Wang, B. & Wood, I. S. Hypoxia and the endocrine and signalling role of white adipose tissue. Arch. Physiol. Biochem. 114, 267–276 (2008).
Ouchi, N., Parker, J. L., Lugus, J. J. & Walsh, K. Adipokines in inflammation and metabolic disease. Nature Rev. Immunol. 11, 85–97 (2011).
Kolonin, M. G., Saha, P. K., Chan, L., Pasqualini, R. & Arap, W. Reversal of obesity by targeted ablation of adipose tissue. Nature Med. 10, 625–632 (2004).
Rupnick, M. A. et al. Adipose tissue mass can be regulated through the vasculature. Proc. Natl Acad. Sci. USA 99, 10730–10735 (2002).
Okuno, A. et al. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J. Clin. Invest. 101, 1354–1361 (1998).
De Souza, C. J. et al. Effects of pioplitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. Diabetes 50, 1863–1871 (2001).
Kim, J. Y. et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117, 2621–2637 (2007).
Yamauchi, T. et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature Med. 7, 941–946 (2001).
De Souza, C. J. et al. Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistence. Diabetes 50, 1863–1871 (2001).
Kusminski, C. M. et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nature Methods 18, 1539–1549 (2012).
Khan, T. et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell. Biol. 29, 1575–1591 (2009).
Jespersen, N. Z. et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 17, 798–805 (2013).
Lidell, M. E. et al. Evidence for two types of brown adipose tissue in humans. Nature Med. 19, 631–634 (2013). Infant interscapular BAT was found to express markers of canonical rodent brown adipocytes, indicating the presence of bona fide brown adipose tissue in humans.
Cypess, A. M. et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nature Med. 19, 635–639 (2013). In this gene expression analysis, adult human neck fat showed enrichment of rodent brown-adipocyte markers and rodent beige-adipocyte markers in distinct locations, suggesting the co-existence of brown and beige-like adipocytes in adult humans.
Cinti, S. et al. Immunohistochemical localization of leptin and uncoupling protein in white and brown adipose tissue. Endocrinology 138, 797–804 (1997).
Ouellet, V. et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J. Clin. Invest. 122, 545–552 (2012).
Lee, P. et al. High prevalence of brown adipose tissue in adult humans. J. Clin. Endocrinol. Metab. 96, 2450–2455 (2011).
Muzik, O. et al. 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat. J. Nucl. Med. 54, 523–531 (2013).
Yoneshiro, T. et al. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring) 19, 13–16 (2011).
Pfannenberg, C. et al. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes 59, 1789–1793 (2010).
Ouellet, V. et al. Outdoor temperture, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake of 18F-FDG-detected BAT in humans. J. Clin. Endocrinol. Metab. 96, 192–199 (2011).
Yoneshiro, T. et al. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring) 19, 1755–1760 (2011).
Symonds, M. E. et al. Thermal imaging to assess age-related changes of skin temperature within the supraclavicular region co-locating with brown adipose tissue in healthy children. J. Pediatr. 161, 892–898 (2012).
van der Lans, A. A. J. J. et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Invest. 123, 3395–3403 (2013).
Yoneshiro, T. et al. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Invest. 123, 3404–3408 (2013).
Vijgen, G. H. E. J. et al. Increased oxygen consumption in human adipose tissue from the “brown adipose tissue” region. J. Clin. Endocrinol. Metab. 98, E1230–E1234 (2013).
Lee, P., Werner, C. D., Kebebew, E. & Celi, F. S. Functional thermogenic beige adipogenesis is inducible in human neck fat. Int. J. Obes. 38, 170–176 (2014).
Sengenès, C., Berlan, M., De Glisezinski, I., Lafontan, M. & Galitzky, J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 14, 1345–1351 (2000).
Lee, P. et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 19, 302–309 (2014).
Lee, P. et al. Mild cold exposure modulates fibroblast growth factor 21 (FGF21) diurnal rhythm in humans: relationship between FGF21 levels, lipolysis, and cold-induced thermogenesis. J. Clin. Endocrinol. Metab. 98, E98–E102 (2013).
Ahfeldt, T. et al. Programming human pluripotent stem cells into white and brown adipocytes. Nature Cell Biol. 14, 209–219 (2012).
Nishio, M. et al. Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell Metab. 16, 394–406 (2012). Refs 122 and 123 are pioneering studies in which brown adipocytes were generated from human embryonic stem cells and human induced pluripotent stem cells.
Gunawardana, S. C. & Piston, D. W. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes 61, 674–682 (2012).
Stanford, K. I. et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Invest. 123, 215–223 (2013).
Acknowledgements
We apologize to those whose work was not directly cited because of space constraints. The authors are supported by FP7 BetaBAT, BBSRC, BHF and MRC programme grants, Wellcome Trust, and Cambridge Overseas Trust.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reprints and permissions information is available at www.nature.com/reprints.
Supplementary information
Rights and permissions
About this article
Cite this article
Peirce, V., Carobbio, S. & Vidal-Puig, A. The different shades of fat. Nature 510, 76–83 (2014). https://doi.org/10.1038/nature13477
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13477
This article is cited by
-
Characteristic and fate determination of adipose precursors during adipose tissue remodeling
Cell Regeneration (2023)
-
Astragalus polysaccharide prevents heart failure-induced cachexia by alleviating excessive adipose expenditure in white and brown adipose tissue
Lipids in Health and Disease (2023)
-
In-vivo detection of white adipose tissue browning: a multimodality imaging approach
Scientific Reports (2023)
-
Hypoxia-induced signaling in the cardiovascular system: pathogenesis and therapeutic targets
Signal Transduction and Targeted Therapy (2023)
-
Transcriptomics profiling reveal the heterogeneity of white and brown adipocyte
Journal of Bioenergetics and Biomembranes (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.