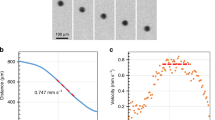
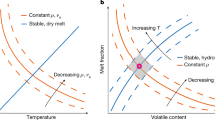
Abstract
The low-viscosity layer in the upper mantle, the asthenosphere, is a requirement for plate tectonics1. The seismic low velocities and the high electrical conductivities of the asthenosphere are attributed either to subsolidus, water-related defects in olivine minerals2,3,4 or to a few volume per cent of partial melt5,6,7,8, but these two interpretations have two shortcomings. First, the amount of water stored in olivine is not expected to be higher than 50 parts per million owing to partitioning with other mantle phases9 (including pargasite amphibole at moderate temperatures10) and partial melting at high temperatures9. Second, elevated melt volume fractions are impeded by the temperatures prevailing in the asthenosphere, which are too low, and by the melt mobility, which is high and can lead to gravitational segregation11,12. Here we determine the electrical conductivity of carbon-dioxide-rich and water-rich melts, typically produced at the onset of mantle melting. Electrical conductivity increases modestly with moderate amounts of water and carbon dioxide, but it increases drastically once the carbon dioxide content exceeds six weight per cent in the melt. Incipient melts, long-expected to prevail in the asthenosphere10,13,14,15, can therefore produce high electrical conductivities there. Taking into account variable degrees of depletion of the mantle in water and carbon dioxide, and their effect on the petrology of incipient melting, we calculated conductivity profiles across the asthenosphere for various tectonic plate ages. Several electrical discontinuities are predicted and match geophysical observations in a consistent petrological and geochemical framework. In moderately aged plates (more than five million years old), incipient melts probably trigger both the seismic low velocities and the high electrical conductivities in the upper part of the asthenosphere, whereas in young plates4, where seamount volcanism occurs6, a higher degree of melting is expected.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Höink, T., Jellinek, A. M. & Lenardic, A. Viscous coupling at the lithosphere-asthenosphere boundary. Geochem. Geophys. Geosyst. 12, Q0AK02 (2011)
Hirth, G. & Kohlstedt, D. L. Water in the oceanic upper mantle: Implications for rheology, melt extraction and the evolution of the lithosphere. Earth Planet. Sci. Lett. 144, 93–108 (1996)
Karato, S. On the origin of the asthenosphere. Earth Planet. Sci. Lett. 321–322, 95–103 (2012)
Evans, R. L. et al. Geophysical evidence from the MELT area for compositional controls on oceanic plates. Nature 437, 249–252 (2005)
Naif, S., Key, K., Constable, S. & Evans, R. L. Melt-rich channel observed at the lithosphere-asthenosphere boundary. Nature 495, 356–359 (2013)
Caricchi, L., Gaillard, F., Mecklenburgh, J. & Le Trong, E. Experimental determination of electrical conductivity during deformation of melt-bearing olivine aggregates: Implications for electrical anisotropy in the oceanic low velocity zone. Earth Planet. Sci. Lett. 302, 81–94 (2011)
Ni, H., Keppler, H. & Behrens, H. Electrical conductivity of hydrous basaltic melts: implications for partial melting in the upper mantle. Contrib. Mineral. Petrol. 162, 637–650 (2011)
Schmerr, N. The Gutenberg discontinuity: melt at the lithosphere-asthenosphere boundary. Science 335, 1480–1483 (2012)
Hirschmann, M., Tenner, T., Aubaud, C. & Withers, A. C. Dehydration melting of nominally anhydrous mantle: the primacy of partitioning. Phys. Earth Planet. Inter. 176, 54–68 (2009)
Green, D. H., Hibberson, W. O., Kovács, I. & Rosenthal, A. Water and its influence on the lithosphere-asthenosphere boundary. Nature 467, 448–451 (2010)
Hier-Majumder, S. & Courtier, A. Seismic signature of small melt fraction atop the transition zone. Earth Planet. Sci. Lett. 308, 334–342 (2011)
Faul, U. H. Melt retention and segregation beneath mid-ocean ridges. Nature 410, 920–923 (2001)
Presnall, D. C. & Gudfinnsson, G. H. Carbonate-rich melts in the oceanic low-velocity zone and deep mantle. Spec. Pap. Geol. Soc. Am. 388, 207–216 (2005)
Wallace, M. E. & Green, D. H. An experimental determination of primary carbonatite magma composition. Nature 335, 343–346 (1988)
Green, D. H. & Liebermann, R. C. Phase-equilibra and elastic properties of a pyrolite model for oceanic upper mantle. Tectonophysics 32, 61–92 (1976)
Fischer, K. M., Ford, H. A., Abt, D. L. & Rychert, C. A. The lithosphere-asthenosphere boundary. Annu. Rev. Earth Planet. Sci. 38, 551–575 (2010)
Hirschmann, M. M. Partial melt in the oceanic low velocity zone. Phys. Earth Planet. Inter. 179, 60–71 (2010)
Utada, H. & Baba, K. Estimating the electrical conductivity of the melt phase of a partially molten asthenosphere from seafloor magnetotelluric sounding data. Phys. Earth Planet. Inter. 227, 41–47 (2013)
Gaillard, F., Malki, M., Iacono-Marziano, G., Pichavant, M. & Scaillet, B. Carbonatite melts and electrical conductivity in the asthenosphere. Science 322, 1363–1365 (2008)
Yoshino, T., Laumonier, M., McIsaac, E. & Katsura, T. Electrical conductivity of basaltic and carbonatite melt-bearing peridotites at high pressures: implications for melt distribution and melt fraction in the upper mantle. Earth Planet. Sci. Lett. 295, 593–602 (2010)
Hirano, N. et al. Volcanism in response to plate flexure. Science 313, 1426–1428 (2006)
Dasgupta, R. et al. Carbon-dioxide-rich silicate melt in the Earth’s upper mantle. Nature 493, 211–215 (2013)
Stagno, V., Ojwang, D. O., McCammon, C. A. & Frost, D. J. The oxidation state of the mantle and the extraction of carbon from Earth’s interior. Nature 493, 84–88 (2013)
Minarik, W. G. & Watson, E. B. Interconnectivity of carbonate melt at low melt fraction. Earth Planet. Sci. Lett. 133, 423–437 (1995)
Jones, A. G., Fullea, J., Evans, R. L. & Muller, M. R. Water in cratonic lithosphere: calibrating laboratory determined models of electrical conductivity of mantle minerals using geophysical and petrological observations. Geochem. Geophys. Geosyst. 13, Q06010 (2012)
Cartigny, P., Pineau, F., Aubaud, C. & Javoy, M. Towards a consistent mantle carbon flux estimate: insights from volatile systematics (H2O/Ce, δD, CO2/Nb) in the North Atlantic mantle (14° N and 34° N). Earth Planet. Sci. Lett. 265, 672–685 (2008)
Marty, B. The origins and concentrations of water, carbon, nitrogen and noble gases on Earth. Earth Planet. Sci. Lett. 313–314, 56–66 (2012)
Dasgupta, R. & Hirschmann, M. M. The deep carbon cycle and melting in Earth's interior. Earth Planet. Sci. Lett. 298, 1–13 (2010)
Lizarralde, D., Chave, A., Hirth, G. & Schultz, A. Northeastern Pacific mantle conductivity profile from long-period magnetotelluric sounding using Hawaii-to-California submarine cable data. J. Geophys. Res. 100 (B9). 17837–17854 (1995)
O’Reilly, S. Y. & Griffin, W. L. The continental lithosphere-asthenosphere boundary: can we sample it? Lithos 120, 1–13 (2010)
Javoy, M. & Pineau, F. The volatiles record of a “popping” rock from the Mid-Atlantic Ridge at 14 ° N: chemical and isotopic composition of gas trapped in the vesicles. Earth Planet. Sci. Lett. 107, 598–611 (1991)
Pommier, A., Gaillard, F., Malki, M. & Pichavant, M. Methodological re-evaluation of the electrical conductivity of silicate melts. Am. Mineral. 95, 284–291 (2010)
Gaillard, F. Laboratory measurements of electrical conductivity of hydrous and dry silicic melts under pressure. Earth Planet. Sci. Lett. 218, 215–228 (2004)
Hashim, L. et al. Experimental assessment of the relationships between electrical resistivity, crustal melting and strain localization beneath the Himalayan-Tibetan belt. Earth Planet. Sci. Lett. 373, 20–30 (2013)
Pommier, A., Gaillard, F., Pichavant, M. & Scaillet, B. Laboratory measurements of electrical conductivities of hydrous and dry Mount Vesuvius melts under pressure. J. Geophys. Res. Solid Earth 113, B05205 (2008)
Tyburczy, J. & Waff, H. S. Electrical conductivity of molten basalt and andesite to 25 kilobars pressure: geophysical significance and implications for the charge transport and melt structure. J. Geophys. Res. 88, 2413–2430 (1983)
Katz, R. F., Spiegelman, M. & Langmuir, C. H. A new parameterization of hydrous mantle melting. Geochem. Geophys. Geosyst. 4, 1073 (2003)
Keppler, H., Wiedenbeck, M. & Shcheka, S. S. Carbon solubility in olivine and the mode of carbon storage in the Earth’s mantle. Nature 424, 414–416 (2003)
Lange, R. A. & Carmichael, I. S. E. Thermodynamic properties of silicate liquids with emphasis on density thermal expansion and compressibility. Rev. Mineral. 24, 25–64 (1990)
Liu, Q. & Lange, R. A. New density measurements on carbonate liquids and the partial molar volume of the CaCO3 component. Contrib. Mineral. Petrol. 146, 370–381 (2003)
Guillot, B. & Sator, N. Carbon dioxide in silicate melts: a molecular dynamics simulation study. Geochim. Cosmochim. Acta 75, 1829–1857 (2011)
ten Grotenhuis, S. M. & Drury, M. R. Spiers, C. J. & Peach, C. J. Melt distribution in olivine rocks based on electrical conductivity measurements. J. Geophys. Res. 110, B12201 (2005)
Hammouda, T. & Laporte, D. Ultrafast mantle impregnation by carbonatite melts. Geology 28, 283–285 (2000)
Glover, P. W. J., Hole, M. J. & Pous, J. A modified Archie’s law for two-conducting phases. Earth Planet. Sci. Lett. 180, 369–383 (2000)
Partzsch, G. M., Schilling, F. R. & Arndt, J. The influence of partial melting on the electrical behaviour of crustal rocks: laboratory examinations, model calculations and geological interpretations. Tectonophysics 317, 189–203 (2000)
Yoshino, T., McIsaac, E., Laumonier, M. & Katsura, T. Electrical conductivity of partial molten carbonate peridotite. Phys. Earth Planet. Inter. 194–195, 1–9 (2012)
Garapić, G., Faul, U. H. & Brisson, E. High-resolution imaging of the melt distribution in partially molten upper mantle rocks: evidence for wetted two-grain boundaries. Geochem. Geophys. Geosyst. 14, 556–566 (2013)
Green, D. H. & Falloon, T. J. Primary magmas at mid-ocean ridges, “hotspots,” and other intraplate settings: constraints on mantle potential temperature. Spec. Pap. Geol. Soc. Am. 388, 217–247 (2005)
Katz, R. F. & Weatherley, S. M. Consequences of mantle heterogeneity for melt extraction at mid-ocean ridges. Earth Planet. Sci. Lett. 335-336, 226–237 (2012)
Hier-Majumder, S., Ricard, Y. & Bercovici, D. Role of grain boundaries in magma migration and storage. Earth Planet. Sci. Lett. 248, 735–749 (2006)
Takei, Y. & Holtzman, B. K. Viscous constitutive relations of solid-liquid composites in terms of grain boundary contiguity: 1. Grain boundary diffusion control model. J. Geophys. Res. 114, B06205 (2009)
Sakamaki, T. et al. Ponded melt at the boundary between the lithosphere and asthenosphere. Nat. Geosci. 6, 1041–1044 (2013)
Hirschmann, M. M. Mantle solidus: experimental constraints and the effects of peridotite composition. Geochem. Geophys. Geosyst. 1, 1042 (2000)
Dasgupta, R. Ingassing, storage, and outgassing of terrestrial carbon through geologic time. Rev. Mineral. Geochem. 75, 183–229 (2013)
Iacono-Marziano, G., Morizet, Y., Le Trong, E. & Gaillard, F. New experimental data and semi-empirical parameterization of H2O-CO2 solubility in mafic melts. Geochim. Cosmochim. Acta 97, 1–23 (2012)
Acknowledgements
This work, part of the ElectroLith project, benefited from funding by the European Research Council (ERC project #279790) and the French agency for research (ANR project #2010 BLAN62101). S.H.-M. acknowledges support from the US NSF grant EAR1215800 and a grant from the University of Orleans. We thank David H. Green for comments.
Author information
Authors and Affiliations
Contributions
F.G. led the project and wrote the first draft. All authors contributed equally to the writing of subsequent drafts. D.S. and F.G. developed the experimental set-up, and D.S. performed the conductivity measurements. S.H.-M. contributed to the discussion and provided editorial assistance with the manuscript. D.S. and L.H. produced Fig. 1, E.G. and L.H. produced Fig. 2, D.S. produced Fig. 3, and L.H. and M.M. produced Fig. 4.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Set-up of electrical conductivity measurement using four wires.
a, Modified piston–cylinder assembly for electrical conductivity measurements using a four-wire configuration. The cored sample (in green) contains in its centre an inner electrode in platinum (in blue). A platinum foil (in blue) surrounds the sample, which extends upwards and downwards from the sample and corresponds to the outer electrode. The sample is sandwiched by machined MgO ceramics (in white). The electrode-sample assemblage is isolated from the graphite furnace by an Al2O3 jacket (in yellow). The four-electrode wires are emplaced using a four-hole Al2O3 tube (in orange). Two of these wires, that is, the thermocouple, are in contact with the inner electrode, whereas the outer electrode is in contact with two other wires by means of a top Ni plug (in red). b, SEM image of the assemblage of sample C after experiments (up to 1,463 °C and 3 GPa). We observed an average decrease of 20% compared to the initial cell geometry (corresponding to the porosity loss during melting). Cell geometry parameters (h, rin and rout in equation (2)) are determined from SEM images for each sample.
Extended Data Figure 2 Measured resistance of molten carbonate versus nickel.
a, The electrical cell resistance versus temperature. We show the resistance of a sample made of nickel measured using either a two-wire set-up (empty diamond) or a four-wire set-up (red diamond). There are several orders of magnitude of difference between the two measurements, showing that the two-wire setup is not suitable at all for conductive materials. We also show the resistance of carbonate in a four-wire set-up (sample C, molten at T > 1,230 °C; green triangle). b, Impedance spectra obtained on molten carbonate (sample C) at 3 GPa as a function of temperature. Impedance spectra show vertical lines, indicating an inductance-dominated signal for all temperatures. The resistance is taken from the intercept with the horizontal axis. Data are obtained at frequencies ranging from 19,905 to 315,479 Hz. The black line represents an impedance spectrum of a nickel sample (blank) obtained with a four-wire configuration at 1,464 °C.
Extended Data Figure 3 Electrical conductivity measurements.
a, Electrical conductivity versus reciprocal temperature measured on carbonated melts and hydrous carbonated basalts. Samples: a carbonated melt (C), a hydrous carbonated melt (HC), and three hydrous carbonated basalts with H2O contents ranging from 4.43 to 9.22 wt% (HCB-9, HCB-7 and HCB-4) and CO2 contents ranging from 10.39 to 23.32 wt%. To complete Fig. 1, we distinguished heating–cooling temperature cycles and reported error bars. Large solid symbols, heating cycle (H1); open symbols, cooling cycle (C1); small solid symbols, second heating cycle (H2) (compare with Extended Data Table 2a). The error bars include uncertainties in the geometrical factors of the samples and in the measured resistance. b, Compensation plots showing the correlation between activation energy, Ea, and pre-exponential terms, ln(σ0). Hydrous basalts (HB) are from the experimental data set of ref. 7 between 1,200 and 1,500 °C, and the data point for the dry basalt (B) is from ref. 32. The dry carbonated melt (C), the hydrous carbonated melts (HC) and the hydrous carbonated basalts (HCB) are from this study (see Extended Data Table 2b for the Arrhenius parameters).
Extended Data Figure 4 The incipient melt effect on the electrical conductivity of an H2O-enriched, CO2-free peridotite.
This figure completes the scenarios illustrated in Fig. 2. The conductivity of partially molten peridotite, in which H2O partitions between minerals and melt (Methods), is reported as a function of melt content and temperature for CO2-free peridotite with 500 p.p.m. H2O (log values; conductivity increases from cold to warm colours). The discontinuity at T = 1,070 °C is due to pargasite amphibole breakdown (Extended Data Fig. 5) that redistribute H2O between NAMs and the melt as explained in the Methods. Melt H2O contents (blue if pargasite out, green if pargasite in) are tabulated above the panel.
Extended Data Figure 5 Melting curves for different bulk peridotitic systems as functions of temperature and depth.
The solidus of dry peridotite (black curve) is calculated from ref. 53. The dehydration solidus of nominally anhydrous peridotite at 200 p.p.m. H2O (blue curve) is modelled from ref. 9. The dehydration solidus of pargasite lherzolite is based on ref. 10. The nominally anhydrous carbonated, fertile peridotite solidus is based on ref. 54 and references therein (green curve). The H2O-undersaturated carbonated, fertile peridotite curve (purple curve) corresponds to the solidus of a pyrolite with 0.5–2.5 wt% CO2 and 0.3 wt% H2O (ref. 14). For pressures ≤1.7 GPa, carbonated melts are unstable and gaseous CO2 prevails. We connected the melting curve of CO2-bearing peridotite to that of the dry peridotite at low pressures, which slightly differs from previously published phase diagrams. We considered that, for P ≤ 1.7 GPa, gaseous CO2 must have a negligible influence on the peridotite solidus due to the small solubility of CO2 in basaltic melts55. Similarly, at low pressures, the H2O-undersaturated carbonated, fertile peridotite solidus was connected to the dehydration solidus of nominally anhydrous peridotite (considering peridotite with 200 p.p.m. H2O), neglecting the presence of pargasite owing to the NAM’s H2O capacity storage.
Extended Data Figure 6 Phase equilibria control on H2O–CO2 partitioning, ultimately resulting in a change in conductivity as shown in Fig. 3.
We show changes in H2O content in olivine (left), melt fraction (centre) and melt CO2/H2O (right) for the 70-Myr age used for calculation in Fig. 3. We use two illustrative compositions: bulk with 200 p.p.m. H2O and 500 p.p.m. CO2 and bulk with 500 p.p.m. H2O and 500 p.p.m. CO2.
Rights and permissions
About this article
Cite this article
Sifré, D., Gardés, E., Massuyeau, M. et al. Electrical conductivity during incipient melting in the oceanic low-velocity zone. Nature 509, 81–85 (2014). https://doi.org/10.1038/nature13245
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13245
This article is cited by
-
The Pannon LitH2Oscope magnetotelluric array in the Pannonian Basin
Acta Geodaetica et Geophysica (2024)
-
Carbonatite-melilitite-phosphate immiscible melts from the aragonite stability field entrained from the mantle by a Pliocene basalt
Mineralogy and Petrology (2023)
-
The mantle structure below petit-spot volcanoes
Communications Earth & Environment (2022)
-
A plume origin for hydrous melt at the lithosphere–asthenosphere boundary
Nature (2022)
-
Preparation of Al2O3–Cr2O3 Solid Solutions as Buoyancy Markers and Their High Pressure Synchrotron X-ray Diffraction Analysis
Pure and Applied Geophysics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.