Abstract
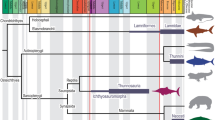
Odontocetes (toothed whales, dolphins and porpoises) hunt and navigate through dark and turbid aquatic environments using echolocation; a key adaptation that relies on the same principles as sonar1. Among echolocating vertebrates, odontocetes are unique in producing high-frequency vocalizations at the phonic lips, a constriction in the nasal passages just beneath the blowhole, and then using air sinuses and the melon to modulate their transmission2,3. All extant odontocetes seem to echolocate2,4; however, exactly when and how this complex behaviour—and its underlying anatomy—evolved is largely unknown. Here we report an odontocete fossil, Oligocene in age (approximately 28 Myr ago), from South Carolina (Cotylocara macei, gen. et sp. nov.) that has several features suggestive of echolocation: a dense, thick and downturned rostrum; air sac fossae; cranial asymmetry; and exceptionally broad maxillae. Our phylogenetic analysis places Cotylocara in a basal clade of odontocetes, leading us to infer that a rudimentary form of echolocation evolved in the early Oligocene, shortly after odontocetes diverged from the ancestors of filter-feeding whales (mysticetes). This was followed by enlargement of the facial muscles that modulate echolocation calls, which in turn led to marked, convergent changes in skull shape in the ancestors of Cotylocara, and in the lineage leading to extant odontocetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Au, W. W. L. The Sonar of Dolphins (Springer-Verlag, 1993)
Cranford, T. W., Amundin, M. & Norris, K. S. Functional morphology and homology in the odontocete nasal complex: implications for sound generation. J. Morphol. 228, 223–285 (1996)
Cranford, T. W. et al. Observation and analysis of sonar signal generation in the bottlenose dolphin (Tursiops truncatus): evidence for two sonar sources. J. Exp. Mar. Biol. Ecol. 407, 81–96 (2011)
Ketten, D. R. in Marine Mammal Sensory Systems (eds Thomas, J.A., Kastelein, R.A. & Supin, A.Y. ) 53–75 (Plenum Press, 1992)
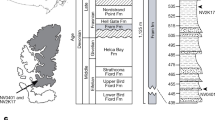
Sanders, A. E., Weems, R. E., Lemon, E. M. & Jr Chandler Bridge Formation—a new Oligocene stratigraphic unit in the lower coastal plain of South Carolina. US Geol. Surv. Bull. 1529, 105–124 (1982)
Uhen, M. D. A new Xenorophus-like odontocete cetacean from the Oligocene of North Carolina and a discussion of the basal odontocete radiation. J. Syst. Palaeontology 6, 433–452 (2008)
McKenna, M. F., Cranford, T. W., Berta, A. & Pyenson, N. Morphology of the odontocete melon and its implications for acoustic function. Mar. Mamm. Sci. 28, 690–713 (2012)
Reidenberg, J. S. & Laitman, J. T. Sisters of the sinuses: cetacean air sacs. Anat. Rec (Hoboken) 291, 1389–1396 (2008)
Mead, J. G. Anatomy of the external nasal passages and facial complex in the Delphinidae (Mammalia: Cetacea). Smithson. Contrib. Zool. 207, 1–35 (1975)
Fordyce, R. E. in Cenozoic Mammals of Land and Sea: Tributes to the Career of Clayton E. Ray (ed. Emry, R. J. ) 185–222 (Smithsonian Institution Scholarly Press, 2002)
Barnes, L. G., Goedert, J. L. & Furusawa, H. in Western Association of Vertebrate Paleontologists with Mesa Southwest Museum and Southwest Paleontological Society: First Meeting of the New Millenium Vol. 8 (eds McCord, R. D. & Boaz, D. ) 91–100 (Southwest Paleontological Society, 2001)
Fraser, F. C. & Purves, P. E. Hearing in cetaceans, evolution of the accessory air sacs and the structure and function of the outer and middle ear in recent cetaceans. Bull. Brit. Mus. Nat. Hist. Zool. 7, 1–140 (1960)
Huggenberger, S., Rauschmann, M. A., Vogl, T. J. & Oelschläger, H. H. Functional morphology of the nasal complex in the harbor porpoise (Phocoena phocoena L.). Anat. Rec. (Hoboken) 292, 902–920 (2009)
Fordyce, E. & de Muizon, C. in Secondary Adaptation of Tetrapods to Life in Water (eds Mazin, J.-M. & de Buffrénil, V. ) 169–234 (Verlag Dr. Friedrich Pfeil, 2001)
Heyning, J. E. Comparative facial anatomy of beaked whales (Ziphiidae) and a systematic revision among the families of extant Odontoceti. Nat. Hist. Mus. Los Angeles Cty. Contrib. Sci. 405, 1–64 (1989)
Carte, A. & Macalister, A. On the anatomy of Balaenoptera rostrata. Phil. Trans. R. Soc. Lond. 158, 201–261 (1868)
Madsen, P. T., Wisniewska, D. & Beedholm, K. Single source sound production and dynamic beam formation in echolocating harbor porpoises (Phocoena phocoena). J. Exp. Biol. 213, 3105–3110 (2010)
Macleod, C. D. et al. Breaking symmetry: the marine environment, prey size, and the evolution of asymmetry in cetacean skulls. Anat. Rec. (Hoboken) 290, 539–545 (2007)
Fahlke, J. M., Gingerich, P. D., Welsh, R. C. & Wood, A. R. Cranial asymmetry in Eocene archaeocete whales and the evolution of directional hearing in water. Proc. Natl Acad. Sci. USA 108, 14545–14548 (2011)
Norberg, R. A. in Ecology and Conservation of Owls (eds Newton, I., Kavanagh, R., Olsen, J. & Taylor, I. ) 329–342 (Csiro Publishing, 2002)
Martínez-Cáceres, M. & de Muizon, C. A new basilosaurid (Cetacea, Pelagiceti) from the late Eocene to early Oligocene Otuma Formation of Peru. C. R. Palevol 10, 517–526 (2011)
Cranford, T. W., Krysl, P. & Hildebrand, J. A. Acoustic pathways revealed: simulated sound transmission and reception in Cuvier’s beaked whale (Ziphius cavirostris). Bioinspir. Biomim. 3, 016001 (2008)
McGowen, M. R., Spaulding, M. & Gatesy, J. Divergence date estimation and a comprehensive molecular tree of extant cetaceans. Mol. Phylogenet. Evol. 53, 891–906 (2009)
Geisler, J. H., McGowen, M. R., Yang, G. & Gatesy, J. A supermatrix of genomic, morphological, and paleontological data from crown Cetacea. BMC Evol. Biol. 11, 112 (2011)
Geisler, J. H., Godfrey, S. J. & Lambert, O. A new genus and species of late Miocene inioid (Cetacea, Odontoceti) from the Meherrin River, North Carolina, U.S.A. J. Vertebr. Paleontol. 32, 198–211 (2012)
Gatesy, J. et al. A phylogenetic blueprint for a modern whale. Mol. Phylogenet. Evol. 66, 479–506 (2013)
Geisler, J. H. & Sanders, A. E. Morphological evidence for the phylogeny of Cetacea. J. Mamm. Evol. 10, 23–129 (2003)
Goloboff, P. A., Farris, J. S. & Nixon, K. C. TNT, a free program for phylogenetic analysis. Cladistics 24, 774–786 (2008)
Fordyce, R. E. in Contributions in Marine Mammal Paleontology Honoring Frank Whitmore Jr (eds Berta, A. & Deméré, T. A. ) 147–176 (San Diego Society of Natural History, 1994)
Dubrovo, I. A. & Sanders, A. E. A new species of Patriocetus (Mammalia, Cetacea) from the late Oligocene of Kazakhstan. J. Vertebr. Paleontol. 20, 577–590 (2000)
Acknowledgements
This research was supported by funds from the New York Institute of Technology, College of Charleston, and M. Brown. During this project we benefitted from discussions with B. Beatty, P. Gingerich, W. Hillenius, M. Mihlbachler, A. Sanders and N. Solounias. We thank A. Sanders and The Charleston Museum for access to specimens under their care. B. Dulguun drew the skulls in Fig. 3. We acknowledge the Deptartment of Radiology, Medical University of South Carolina, and T. Holden in particular, for conducting CT scans of Cotylocara. We thank T. Rowe for access to the Tursiops scan data, which were scanned using his NSF digital libraries initiative grant (IIS-987-4781).
Author information
Authors and Affiliations
Contributions
J.H.G. designed the research plan and conducted the phylogenetic analysis. M.W.C. generated three-dimensional models from CT data and conducted related analyses on this data. J.H.G. wrote the paper with discussion from J.L.C and M.W.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Supermatrix used for phylogenetic analysis deposited at Morphobank (http://www.morphobank.org/) project P888. The new taxa (new genus and new species) have been registered with Zoobank (LSID: urn:lsid:zoobank.org:pub:415D35BC-3306-4361-B34D-B7702649B425).
Extended data figures and tables
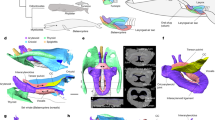
Extended Data Figure 1 Images of the holotype skull of Cotylocara macei (CCNHM-101).
a, Parasagittal section of the skull generated from CT slices demonstrating the anatomical basis for klinorhynchy (downturned face and rostrum). Red lines follow the axis of the basicranial stem and the rostrum; together they form an angle of approximately 20°. This slice is situated <10 mm to the right of the median plane. Other slices, medial and lateral to this one, display similar angles. b, Medial view of the right dentary. Bo, basioccipital; Bs, basisphenoid; cc, cranial cavity; co, mandibular condyle; cp, coronoid process; ip, interparietal; mf, mandibular foramen; ms, surface for mandibular symphysis; Mx, Maxilla; Na, nasal; n-1, penultimate tooth; n-5, fifth from the last tooth; Pa, parietal; pf, postnarial fossa; Px, premaxilla; So, supraoccipital; xn, external bony nares.
Extended Data Figure 2 Petrosal and tympanic bones of the holotype skull of Cotylocara macei (CCNHM-101).
a, Ventrolateral view of right petrosal articulated with the rest of the skull. Anterior is towards the upper left corner. b–d, Ventral, dorsal and lateral views of tympanic bulla. e–h, Ventrolateral, dorsomedial, dorsolateral and ventromedial views of right petrosal (periotic). Scale bars are 1 cm. ap, anterior process; bc, basioccipital crest; ca, aperture for cochlear aqueduct; ch, cranial hiatus; ctp, caudal tympanic process; dc, dorsal crest; eam, external acoustic meatus; fc, proximal opening of facial canal; fer, fenestra rotunda; fp, falciform process of squamosal; fi, fossa incudis; fm, mallear fossa; fo, fenestra ovalis; gf, glenoid fossa; he, epitympanic hiatus; iv, involucrum; mf, median furrow; opp, outer posterior prominence; pap, paroccipital process; pc, pars cochlearis; pgp, postglenoid process; pp, posterior process; sf, fossa for stapedial muscle; smf, suprameatal fossa; spf, fossa for sigmoid process; tc, tympanic cavity; tf, articular facet for posterior process of tympanic; va, aperture for vestibular aqueduct; vlt, ventrolateral tuberosity.
Extended Data Figure 3 Postcrania of holotype of Cotylocara macei (CCNHM-101).
a, b, Posteromedial views of anterior left ribs. c, d, Middle left ribs. e, Posterior left rib. f, g, Middle right ribs. h, i, Axis vertebra (C2) in anterior and posterior views. j, Middle cervical (C3 or C4) vertebra in anterior view. k, Sixth cervical vertebra in anterior view. Scale bar for a–g is 5 cm; those for h–k are 1 cm.
Extended Data Figure 4 Holotype of skull of Cotylocara macei (CCNHM-101).
a, Dorsal view. b, Ventral view. Colours represent individual bones of the skull: turquoise, occipital; brown, nasals; grey, epoxy putty; light green, lacrimal; olive, sphenoid; orange, parietals; pink, premaxillae; purple, palatines (ventral side only); red, petrosals; light blue, frontals; dark blue, squamosals; yellow, maxillae; lime green, pterygoids; white, interparietal. alv, alveolus; an, antorbital notch; ap, anterior process of petrosal; apl, ascending process of lacrimal; apm, ascending process of maxilla; asf?, possible air sinus fossa; cc, cranial cavity; df, deep fossa within the broader periotic fossa; dif, dorsal infraorbital foramina; dta, anteriormost double-rooted tooth; dtp, posteriormost double-rooted tooth; ep, embrasure pit; fp, falciform process of squamosal; fw, frontal window; gf, glenoid fossa; gpf, greater palatine foramen; itc, infratemporal crest; nc, nuchal crest; npp, nasal process of premaxilla; pap, paroccipital process; pf, postnarial fossa; pgp, postglenoid process; plp, palatine process of premaxilla; pop, postorbital process of frontal; ppf, preorbital process of frontal; pr, postorbital ridge; rb, rostral basin; ps, palatine sulcus; rc, reconstructed tooth; rif, reentrant infraorbital foramen; spf, fossa for sigmoid process of tympanic; sqf; squamosal fossa; tif, thin lamina of frontal; vlt, ventrolateral tuberosity of petrosal; V3, path of mandibular division of trigeminal nerve; zy, zygomatic process. Boundaries between bones are based on external sutures and CT data. Synchondrosis between basioccipital and basisphenoid is completely closed, thus the boundary shown here is speculative. The frontal–parietal suture is damaged and is our best interpretation. Scale bar is 5 cm.
Extended Data Figure 5 Fossae in the holotype skull of Cotylocara macei (CCNHM-101) that are likely to have been filled with air sinuses.
a, b, CT-generated model of skull in oblique dorsolateral view. c, d, Same as a, b, but from a more dorsal perspective, anterior is towards the lower left corner. Light blue, excavations of an air sinus that may be homologous to the inferior vestibule as well as the route by which it connected to the soft tissue nasal passages. Red, premaxillary air sinus. La, lacrimal; Mx, maxilla; Na, nasal; Pa, parietal; pf, postnarial fossa; Px, premaxilla; rb, rostral basin; So, supraoccipital; Sq, squamosal; zy, zygomatic process.
Extended Data Figure 6 Comparison between the relative densities of bones in the holotype skull of Cotylocara macei (CCNHM-101) and the modern bottlenose dolphin Tursiops truncatus (SDSNH 21212).
a, b, Dorsal and ventral views of a three-dimensional digital model of the holotype skull of Cotylocara macei (CCNHM-101), based on CT data. Densest bones are in bright orange, most porous bones are in purple, and reconstructed portions are in grey. Note the dense rostrum, the less dense bone flooring the rostral basin and postnarial fossae, and the density difference between the dorsal and ventral sides of the skull. c, d, Dorsal and ventral views of a three-dimensional digital model of Tursiops truncatus (SDSNH 21212). Note the porous bone in the premaxillary sac fossa and the similar densities of the dorsal and ventral sides of the skull. pf, postnarial fossa; psf, premaxillary sac fossa; Px, premaxilla; rb, rostral basin; SDSNH, San Diego Society of Natural History. CT scans of Tursiops were performed at the University of Texas High-Resolution X-ray CT Facility (data courtesy of T. Rowe). The voxel size for these data was 0.298 mm × 0.298 mm × 0.9 mm.
Extended Data Figure 7 Evolution of skeletal features likely associated with echolocation.
a, Skull of Georgiacetus vogtlensis in dorsal view: to the left are conditions of characters mapped in d–h and to the right are the different possible character states for posterior migration of the maxilla (yellow) across all taxa. b, c, Skulls of Cotylocara macei and Simocetus rayi in dorsal view, the latter redrawn10, with their respective states for characters mapped in d–h. d–h, Evolution of characters that are likely to be associated with echolocation on our phylogenetic tree (Fig. 3). i, Summary of all changes detailed in d–h with convergent characters in green and non-convergent changes in black; numbers refer to characters described in the Supplementary Information. In a–f and h red is the primitive state, blue is the most derived state, and purple is an intermediate derived state. Shades of grey in g correspond to positions of the maxilla, as illustrated on the right side of a, with changes between character states (states 0 through 5) labelled below for clarity. Dashed terminal branches indicate that data are missing for that fossil taxon. Character evolution was inferred using parsimony on the entire phylogeny, although d–i only include those taxa needed to convey the pattern of character evolution at the base of Odontoceti. All evolutionary reconstructions are unequivocal except for f, which is an ACCTRAN optimization. An alternative, but equally parsimonious reconstruction for f, is discussed in the Supplementary Information. An arrow in d–i indicates where echolocation is inferred to have evolved based on its occurrence in bolded taxa: osteological evidence in Cotylocara macei and observational evidence in the crown group of Odontoceti. More details on the characters and their states can be found in the Supplementary Information.
Supplementary information
Supplementary Information
This file contains Supplementary Notes, a Supplementary Discussion and Supplementary Table 1. (PDF 2381 kb)
Rights and permissions
About this article
Cite this article
Geisler, J., Colbert, M. & Carew, J. A new fossil species supports an early origin for toothed whale echolocation. Nature 508, 383–386 (2014). https://doi.org/10.1038/nature13086
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13086
This article is cited by
-
Ecomorphology of toothed whales (Cetacea, Odontoceti) as revealed by 3D skull geometry
Journal of Mammalian Evolution (2023)
-
Late Miocene Survival of a Hyper-Longirostrine Dolphin and the Neogene to Recent Evolution of Rostrum Proportions Among Odontocetes
Journal of Mammalian Evolution (2022)
-
Wonky whales: the evolution of cranial asymmetry in cetaceans
BMC Biology (2020)
-
Convergent evolution in toothed whale cochleae
BMC Evolutionary Biology (2019)
-
Problematic archaic whale Phococetus (Cetacea: Odontoceti) from the Lee Creek Mine, North Carolina, USA, with comments on geochronology of the Pungo River Formation
PalZ (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.