Abstract
Extant vertebrates form two clades, the jawless Cyclostomata (lampreys and hagfishes) and the jawed Gnathostomata (all other vertebrates), with contrasting facial architectures1,2. These arise during development from just a few key differences in the growth patterns of the cranial primordia: notably, the nasal sacs and hypophysis originate from a single placode in cyclostomes but from separate placodes in gnathostomes, and infraoptic ectomesenchyme migrates forward either side of the single placode in cyclostomes but between the placodes in gnathostomes3,4,5,6,7,8. Fossil stem gnathostomes preserve cranial anatomies rich in landmarks that provide proxies for developmental processes and allow the transition from jawless to jawed vertebrates to be broken down into evolutionary steps7,9,10,11,12. Here we use propagation phase contrast synchrotron microtomography to image the cranial anatomy of the primitive placoderm (jawed stem gnathostome) Romundina13, and show that it combines jawed vertebrate architecture with cranial and cerebral proportions resembling those of cyclostomes and the galeaspid (jawless stem gnathostome) Shuyu11. This combination seems to be primitive for jawed vertebrates, and suggests a decoupling between ectomesenchymal growth trajectory, ectomesenchymal proliferation, and cerebral shape change during the origin of gnathostomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
26 March 2014
Affiliation 3 was updated, and details of scale bars were added to the legends of Extended Data Figs 3 and 4.
References
Kuratani, S. & Horigome, N. Development of peripheral nerves in a cat shark, Scyliorhinus torazame, with special reference to rombomeres, cephalic mesoderm, and distribution patterns of crest cells. Zoolog. Sci. 17, 893–909 (2000)
Kuratani, S., Horigome, N. & Hirano, S. Developmental morphology of the head mesoderm and re-evaluation of segmental theories of the vertebrate head: evidence from embryos of an agnathan vertebrate, Lampetra japonica. Dev. Biol. 210, 381–400 (1999)
Kuratani, S. Evolution of the vertebrate jaw: comparative embryology and molecular developmental biology reveal the factors behind evolutionary novelty. J. Anat. 205, 335–347 (2004)
Kuratani, S. Developmental studies of the lamprey and hierarchical evolutionary steps towards the acquisition of the jaw. J. Anat. 207, 489–499 (2005)
Kuratani, S. Evolution of the vertebrate jaw from developmental perspectives. Evol. Dev. 14, 76–92 (2012)
Kuratani, S., Nobusada, Y., Horigome, N. & Shigetani, Y. Embryology of the lamprey and evolution of the vertebrate jaw: insights from molecular and developmental perspectives. Phil. Trans. R. Soc. Lond. B 356, 1615–1632 (2001)
Oisi, Y., Ota, K. G., Kuraku, S., Fujimoto, S. & Kuratani, S. Craniofacial development of hagfishes and the evolution of vertebrates. Nature 493, 175–180 (2013)
Ota, K. G. & Kuratani, S. Cyclostome embryology and early evolutionary history of vertebrates. Integr. Comp. Biol. 47, 329–337 (2007)
Brazeau, M. D. The braincase and jaws of a Devonian ‘acanthodian’ and modern gnathostome origins. Nature 457, 305–308 (2009)
Davis, S. P., Finarelli, J. A. & Coates, M. I. Acanthodes and shark-like conditions in the last common ancestor of modern gnathostomes. Nature 486, 247–250 (2012)
Gai, Z., Donoghue, M. J., Zhu, M., Janvier, P. & Stampanoni, M. Fossil jawless fish from China foreshadows early jawed vertebrate anatomy. Nature 476, 324–327 (2011)
Zhu, M. et al. A Silurian placoderm with osteichthyan-like marginal jaw bones. Nature (2013)
Ørvig, T. in Problèmes Actuels de Paléontologie: Evolution des Vertébrés (ed. Lehman, J. P. ) 41–72 (Colloques Internationaux Centre National de la Recherche Scientifique, 1975)
Couly, G. F., Coltey, P. M. & Le Douarin, N. M. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development 117, 409–429 (1993)
Janvier, P. Early Vertebrates Vol. 1 (Clarendon, 1996)
Goujet, D. & Young, G. C. Interrelationships of placoderms revisited. Geobios. 28, 89–95 (1995)
Denison, R. H. Placodermi (Gustav Fischer, 1978)
Young, G. C. A new Early Devonian placoderm from New South Wales, Australia, with a discussion of placoderm phylogeny. Palaeontographica 167, 10–76 (1980)
Young, G. C. Reconstruction of the jaws and braincase in the Devonian placoderm fish Bothriolepis. Palaeontology 27, 635–661 (1984)
Goujet, D. & Young, G. C. in Recent Advances in the Origin and Early Radiation of Vertebrates (eds Arratia, G., Wilson, M. V. H. & Cloutier, R. ) 109–126 (Dr. Friedlich Pfeil, 2004)
Dupret, V. Revision of the genus Kujdanowiaspis Stensiö, 1942 (Placodermi, Arthrodira, “Actinolepida”) from the Lower Devonian of Podolia (Ukraine). Geodiversitas 32, 5–63 (2010)
Goujet, D. Les Poissons Placodermes du Spitsberg: Arthrodires Dolichothoraci de la Formation de Wood Bay (Devonien Inferieur) (CNRS, 1984)
Stensiö, E. in Traité de Paléontologie Vol. 4 (ed. Piveteau, J. ) 71–693 (Masson, 1969)
Osumi-Yamashita, N. et al. Cranial anomaly of homozygous rSey rat is associated with a defect in the migration pathway of midbrain crest cells. Dev. Growth Differ. 39, 53–67 (1997)
Wada, N., Nohno, T. & Kuratani, S. Dual origin of the prechordal cranium in the chicken embryo. Dev. Biol. 356, 529–540 (2011)
Zhu, M. & Janvier, P. A small antiarch, Minicrania lirouyii gen. et sp. nov from the early Devonian of Qujing, Yunnan (China), with remarks on the antiarch phylogeny. J. Vertebr. Paleontol. 16, 1–15 (1996)
Lu, J. et al. The earliest known stem-tetrapod from the Lower Devonian of China. Nature Commun. 3, 1160 (2012)
Dupret, V., Sanchez, S., Goujet, D., Tafforeau, P. & Ahlberg, P. Bone vascularization and growth in placoderms (Vertebrata): the example of the premedian plate of Romundina stellina Ørvig, 1975. C. R. Palevol 9, 369–375 (2010)
Collin, S. P. in Fish Physiology (eds McKenzie, D. J., Farell, A. P. & Brauner, C. J. ) 121–179 (Elsevier, 2007)
De Iuliis, G. & Pulerà, D. The Dissection of Vertebrates—A Laboratory Manual (Academic, 2007)
Acknowledgements
We thank the European Synchrotron Radiation Facility for granting us beam time at ID19 (proposal EC-203). P.E.A., V.D. and S.S. acknowledge the support of European Research Council Advanced Investigator Grant 233111 and a Wallenberg Scholarship from the Knut and Alice Wallenberg Foundation, both awarded to P.E.A. We thank B. Ryll, M. Kundrat and H. Blom at Uppsala University for discussions. Specimen MNHN CPW 1 photographs were taken by P. Loubry (Centre National pour la Recherche Scientifique, Muséum national d’Histoire naturelle, Paris).
Author information
Authors and Affiliations
Contributions
The project was conceived by V.D., P.E.A. and S.S. Specimens of Romundina were collected by D.G. Scanning and reconstruction of data sets were carried out by S.S. and P.T., with minor contributions to the scanning by V.D. and P.E.A. Three-dimensional modelling, rendering and animation were done by V.D., who also carried out phylogenetic analysis and anatomical interpretation with input from P.E.A. P.E.A. led the comparative developmental interpretation. P.E.A. and V.D. wrote the text. All authors critically reviewed the manuscript and approved the final draft.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Data are deposited online at http://paleo.esrf.eu.
Extended data figures and tables
Extended Data Figure 1 Majority rule (50%) consensus tree of the 17,620 most parsimonious trees from the phylogenetic analysis.
Numbers at nodes (white on black filled circle) indicate nodes for character state changes described in Supplementary Information. Numbers on branches (black on white) indicate the occurrence percentage of these branches in the 17,620 trees described in Supplementary Information. Jawless stem Gnathostomata (‘ostracoderms’) are in turquoise blue, Placodermi in green, Acanthodii in pink, Chondrichthyes in red, and Osteichthyes in blue.
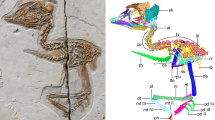
Extended Data Figure 3 Romundina stellina Ørvig, 1975 (13) (specimenMNHNCPW01).
a, b, Skull in anterior oblique view, emphasizing the premedian plate, the nasal region and the orbit (red box magnified in b). c, e, Synchrotron three-dimensional model in lateral (c, red box magnified in d), half-dorsal (e) and half-ventral (f) views. The endocranial cavity and nerves are shown in yellow, perichondral bone in light pink, dermal bone in orange (full transparent in d–f), veins in blue and arteries in red (semi-transparent in d, e). e and f are cut slightly beyond the midline. 0, terminal nerve; I, olfactory nerve; II, optic nerve; III, oculomotor nerve; IV, trochlear nerve; V.r, trigeminal recess; V1, profundus nerve; V2, second branch of the trigeminal nerve; V3(mn), mandibular branch of the trigeminal nerve 3; V3(mx), maxillary branch of the trigeminal nerve 3; VI, pathetic nerve; VII(hm), hyomandibular branch of the facial nerve; VII(op), opercular branch of the facial nerve; VII(pal)g, groove for the palatine ramus of the facial nerve; ?, palatine nerve (from the trigeminal?); a.c.v, anterior cerebral vein; a.j.v, anterior jugular vein; a.j.v.g, groove for the anterior jugular vein; acv, articulation of the palatoquadrate; art, articulation of the palatoquadrate; asc, anterior semicircular canal; cc, central sensory line groove; cc.a, common carotid artery; e.c, endocranial cavity; e.s, eyestalk; eh.a, epihyal artery; hyp.a, hypophyseal artery; hyp.d, hypophyseal duct; hyp.r, hypophyseal recess; hyp.v, hypophyseal vein; i.c.a, internal carotid artery; ioc, infraorbital sensory line groove; j.v, jugular vein; n.s, nasal sac; olf.b, olfactory bulb; op.a, opercular artery; opht.a, ophtalmic artery; pi, pineal organ; pit.v, pituitary vein; PrM, premedian plate; pse.a, pseudobranchial artery; RoPi, rostropineal plate; soc, supraorbital sensory line groove. Scale bars: a, 10 mm; b–d, 2 mm; e, f, 1 mm. All images are original.
Extended Data Figure 4 Romundina stellina Ørvig, 1975 (13) (specimen MNHN CPW 13).
a–e, Near complete skull roof with rostronasal caspule in articulation. a, b, Specimen in dorsal view (red box magnified in b). c, Specimen in anterior view. d, Specimen in left lateral view. e, Specimen in right lateral view. II.f, optic nerve foramen; V1.f, profundus nerve foramen; IV.f, trochlear nerve foramen; III.f, oculomotor nerve foramen; a.c.v.f, anterior cerebral vein foramen; cc, central sensory line groove; e.s, eyestalk; ioc, infraorbitsal sensory line groove; mpl, middle pitline groove; n.s, nasal sac; pi.f, pineal fontanelle; PrM, premedian plate; RoPi, rostropineal plate; soc, supraorbital sensory line groove; s.p, sensory pit. Photographs of specimen MNHN CPW 13 were taken by D. Goujet. Scale bars: 10 mm. Images are original.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data and Supplementary References. (PDF 1403 kb)
Supplementary Data
This zipped file contains the data matrix in MacClade and NDE file versions. (ZIP 26 kb)
Reconstructed slice data of specimen MNHN CPW 1 from Propagation Phase-Contrast Synchrotron Radiation micro-computerized tomography
Slices are transverse sections, and the video runs from anterior (matrix in front of the tip of the snout) posteriorly toward the otic area (stops at the middle of the inner ear). Generated with Image J 1.47v (64 bits; http://imagej.nih.gov/ij). (MP4 8913 kb)
Internal structures of the skull of Romundina stellina (specimen MNHN CPW 1)
The 3D model (orange: dermal bone; pink: perichondral bone) rises from a photograph of the corresponding specimen. Different transparencies provide an ensemble view of the blood vessel network (red: arteries; blue: veins), nerves and endocranial cavity (yellow), shape of telencephalon, nasal sacs and rostronasal capsule, notochord (maroon), and inner ears (light blue). Notice the palatoquadrate attachment surfaces on the elongate prerostral area, the short telencephalon, ant the anterior position and orientation of the hypophysial recess. Cube edge equals 1 mm. (MP4 7694 kb)
Rights and permissions
About this article
Cite this article
Dupret, V., Sanchez, S., Goujet, D. et al. A primitive placoderm sheds light on the origin of the jawed vertebrate face. Nature 507, 500–503 (2014). https://doi.org/10.1038/nature12980
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12980
This article is cited by
-
Fossil evidence for a pharyngeal origin of the vertebrate pectoral girdle
Nature (2023)
-
Sequence of chondrocranial development in basal anurans—Let’s make a cranium
Frontiers in Zoology (2022)
-
The oldest complete jawed vertebrates from the early Silurian of China
Nature (2022)
-
Neurocranial development of the coelacanth and the evolution of the sarcopterygian head
Nature (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.