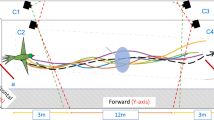
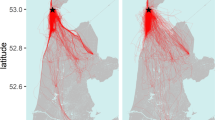
Abstract
Many species travel in highly organized groups1,2,3. The most quoted function of these configurations is to reduce energy expenditure and enhance locomotor performance of individuals in the assemblage4,5,6,7,8,9,10,11. The distinctive V formation of bird flocks has long intrigued researchers and continues to attract both scientific and popular attention4,7,9,10,11,12,13,14. The well-held belief is that such aggregations give an energetic benefit for those birds that are flying behind and to one side of another bird through using the regions of upwash generated by the wings of the preceding bird4,7,9,10,11, although a definitive account of the aerodynamic implications of these formations has remained elusive. Here we show that individuals of northern bald ibises (Geronticus eremita) flying in a V flock position themselves in aerodynamically optimum positions, in that they agree with theoretical aerodynamic predictions. Furthermore, we demonstrate that birds show wingtip path coherence when flying in V positions, flapping spatially in phase and thus enabling upwash capture to be maximized throughout the entire flap cycle. In contrast, when birds fly immediately behind another bird—in a streamwise position—there is no wingtip path coherence; the wing-beats are in spatial anti-phase. This could potentially reduce the adverse effects of downwash for the following bird. These aerodynamic accomplishments were previously not thought possible for birds because of the complex flight dynamics and sensory feedback that would be required to perform such a feat12,14. We conclude that the intricate mechanisms involved in V formation flight indicate awareness of the spatial wake structures of nearby flock-mates, and remarkable ability either to sense or predict it. We suggest that birds in V formation have phasing strategies to cope with the dynamic wakes produced by flapping wings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Couzin, I. D., Krause, J., Franks, N. R. & Levin, S. A. Effective leadership and decision-making in animal groups on the move. Nature 433, 513–516 (2004)
Nagy, M., Akos, Z., Biro, D. & Vicsek, T. Hierarchical group dynamics in pigeon flocks. Nature 464, 890–894 (2010)
May, R. M. Flight formations in geese and other birds. Nature 282, 778–780 (1979)
Lissaman, P. B. & Schollenberger, C. A. Formation flight of birds. Science 168, 1003–1005 (1970)
Liao, J. C., Beal, D. N., Lauder, G. V. & Triantafyllou, M. S. Fish exploiting vortices decrease muscle activity. Science 302, 1566–1569 (2003)
Bill, R. G. & Hernnkind, W. F. Drag reduction by formation movement in spiny lobsters. Science 193, 1146–1148 (1976)
Weimerskirch, H., Martin, J., Clerquin, Y., Alexandre, P. & Jiraskova, S. Energy saving in flight formation. Nature 413, 697–698 (2001)
Fish, F. E. Kinematics of ducklings swimming in formation: consequence of position. J. Exp. Zool. 273, 1–11 (1995)
Badgerow, J. P. & Hainsworth, F. R. Energy savings through formation flight? A re-examination of the vee formation. J. Theor. Biol. 93, 41–52 (1981)
Cutts, C. J. & Speakman, J. R. Energy savings in formation flight of pink-footed geese. J. Exp. Biol. 189, 251–261 (1994)
Hummel, D. Aerodynamic aspects of formation flight in birds. J. Theor. Biol. 104, 321–347 (1983)
Willis, D. J., Peraire, J. & Breuer, K. S. in Proc. 25th American Institute of Aeronautics and Astronautics Appl. Aerodynam. Conf.http://doi.org/10.2514/6.2007-4182. (2007)
Hainsworth, F. R. Precision and dynamics of positioning by Canada geese flying in formation. J. Exp. Biol. 128, 445–462 (1987)
Maeng, J. S. et al. A modelling approach to energy savings of flying Canada geese using computational fluid dynamics. J. Theor. Biol. 320, 76–85 (2013)
Usherwood, J. R., Stavrou, M., Lowe, J. C., Roskilly, K. & Wilson, A. M. Flying in a flock comes at a cost in pigeons. Nature 474, 494–497 (2011)
Wilson, A. M. et al. Locomotion dynamics of hunting in wild cheetahs. Nature 498, 185–189 (2013)
Fisher, N. I. Statistical Analysis of Circular Data Ch. 4, 59–102 (Cambridge Univ. Press, 1993)
Pennycuick, C. J. Bird Flight Performance: A Practical Calculation Manual Ch. 3, 37–78 (Oxford Univ. Press, 1989)
Spence, A. J., Thurman, A. S., Maher, M. J. & Wilson, A. M. Speed, pacing and aerodynamic drafting in thoroughbred horse racing. Biol. Lett. 8, 678–681 (2012)
Chatard, J.-C. & Wilson, B. Drafting distance in swimming. Med. Sci. Sports Exerc. 35, 1176–1181 (2003)
Delextrat, A. et al. Drafting during swimming improves efficiency during subsequent cycling. Med. Sci. Sports Exerc. 35, 1612–1619 (2003)
Kutsch, W., Camhi, J. & Sumbre, G. Close encounters among flying locusts produce wing-beat coupling. J. Comp. Physiol. A 174, 643–649 (1994)
Camhi, J. M., Sumbre, G. & Wendler, G. Wing-beat coupling between flying locusts pairs: preferred phase and life enhancement. J. Exp. Biol. 198, 1051–1063 (1995)
Usherwood, J. R. & Lehmann, F. Phasing of dragonfly wings can improve efficiency by removing swirl. J. R. Soc. Interface 5, 1303–1307 (2008)
White, C. R. et al. Implantation reduces the negative effects of bio-logging on birds. J. Exp. Biol. 216, 537–542 (2013)
King, A. J. et al. Selfish-herd behaviour of sheep under threat. Curr. Biol. 22, R561–R562 (2012)
Barron, D. G., Brawn, J. D. & Weatherhead, P. J. Meta-analysis of transmitter effects on avian behaviour and ecology. Methods Ecol. Evol. 1, 180–187 (2010)
Norberg, U. M. Vertebrate Flight: Mechanics, Physiology, Morphology, Ecology and Evolution Ch. 9, 118–132 (Springer, 2011)
Kaplan, E. & Hegarty, C. Understanding GPS: Principles and Applications Ch. 7, 304–334 (Artech House, 2005)
Andersson, M. & Wallander, J. Kin selection and reciprocity in flight formation? Behav. Ecol. 15, 158–162 (2003)
Petit, D. R. & Bildstein, K. L. Development of formation flying in juvenile white ibises (Eudocimus albus). Auk 103, 244–246 (1986)
Rands, S. A., Cowlishaw, G., Pettifor, R. A., Rowcliffe, J. M. & Johnstone, R. A. Spontaneous emergence of leaders and followers in foraging pairs. Nature 423, 432–434 (2003)
Biro, D., Sumpter, D. J. T., Meade, J. & Guilford, T. From compromise to leadership in pigeon homing. Curr. Biol. 16, 2123–2128 (2006)
Mardia, K. & Jupp, P. Directional Statistics Ch. 6, 94–110 (Wiley, 1999)
Sprent, P. & Smeeton, N. C. Applied Nonparametric Statistical Methods Ch. 4, 83–122 (Taylor & Francis, 2007)
Batschelet, E. Circular Statistics in Biology Chs 9, 15 (Academic, 1981)
Wiltschko, W. et al. Lateralisation of magnetic compass orientation in a migratory bird. Nature 419, 467–470 (2002)
Holland, R. A. et al. Testing the role of sensory systems in the migrating heading of a songbird. J. Exp. Biol. 212, 4065–4071 (2009)
Hubel, T. Y. et al. Wake structure and wing kinematics: the flight of the lesser dog-faced fruit bat, Cynopterus brachyotis. J. Exp. Biol. 213, 3427–3440 (2010)
Hubel, T. Y. et al. Changes in kinematics and aerodynamics over a range of speeds in Tadarida brasiliensis, the Brazilian free-tailed bat. J. R. Soc. Interface 9, 1120–1130 (2012)
Kroner, E. Dislocations and the Biot-Savart law. Proc. Phys. Soc. A 68, 53–55 (1955)
Griffiths, D. J. Introduction to Electrodynamics Ch. 5, 215 (Prentice Hall, 1998)
Acknowledgements
The Waldrappteam assisted with data collection and provided logistical support (J.F., B.V.). We thank members of the Structure & Motion Laboratory for discussions and assistance, particularly J. Lowe, K. Roskilly, A. Spence and S. Amos, and C. White and R. Bomphrey for reading an earlier draft of the paper. Funding was provided by an Engineering and Physical Sciences Research Council grant to A.M.W., J.R.U. and S.Ha. (EP/H013016/1), a Biotechnology and Biological Sciences Research Council grant to A.M.W. (BB/J018007/1) and a Wellcome Trust Fellowship (095061/Z/10/Z) to J.R.U.
Author information
Authors and Affiliations
Contributions
S.J.P., S.Ha., A.M.W. and J.R.U. developed the concept of the paper. J.F., S.He. and D.T. reared and trained the birds. S.J.P., S.He., D.T., B.V. and J.F. collected the field data. S.J.P., T.Y.H. and J.R.U. undertook the data processing and analyses; J.R.U. performed the circular statistics. S.J.P., T.Y.H, A.M.W. and J.R.U wrote the manuscript, with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-8 and Supplementary Table 1. (PDF 4060 kb)
Supplementary Data 1
This zipped file contains a Google EarthTM (Landsat, KML file) image displaying the full flight of the ibis flock, recorded via the 5 Hz GPS data logger. (ZIP 504 kb)
Supplementary Table 2
This file contains full raw data set for phasing analysis (see Methods), covering both the spanwise and streamwise positions. (TXT 17 kb)
A section of the ibis flight
An animated movie showing a section of the ibis flight, taken from the 5 Hz GPS logger data. Each individual bird is identified by a number displayed on the tip of the left wing. (MOV 9838 kb)
Ibis flying behind the paraplane
A short video clip of the ibis flying behind the paraplane during a training flight. (MP4 4772 kb)
Rights and permissions
About this article
Cite this article
Portugal, S., Hubel, T., Fritz, J. et al. Upwash exploitation and downwash avoidance by flap phasing in ibis formation flight. Nature 505, 399–402 (2014). https://doi.org/10.1038/nature12939
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12939
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.