Abstract
Wnts are evolutionarily conserved secreted signalling proteins that, in various developmental contexts, spread from their site of synthesis to form a gradient and activate target-gene expression at a distance. However, the requirement for Wnts to spread has never been directly tested. Here we used genome engineering to replace the endogenous wingless gene, which encodes the main Drosophila Wnt, with one that expresses a membrane-tethered form of the protein. Surprisingly, the resulting flies were viable and produced normally patterned appendages of nearly the right size, albeit with a delay. We show that, in the prospective wing, prolonged wingless transcription followed by memory of earlier signalling allows persistent expression of relevant target genes. We suggest therefore that the spread of Wingless is dispensable for patterning and growth even though it probably contributes to increasing cell proliferation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
van Amerongen, R. & Nusse, R. Towards an integrated view of Wnt signaling in development. Development 136, 3205–3214 (2009)
Clevers, H. & Nusse, R. Wnt/β-catenin signaling and disease. Cell 149, 1192–1205 (2012)
Kiecker, C. & Niehrs, C. A morphogen gradient of Wnt/β -catenin signalling regulates anteroposterior neural patterning in Xenopus. Development 128, 4189–4201 (2001)
Zecca, M., Basler, K. & Struhl, G. Direct and long-range action of a wingless morphogen gradient. Cell 87, 833–844 (1996)
Neumann, C. J. & Cohen, S. M. Long-range action of Wingless organizes the dorsal-ventral axis of the Drosophila wing. Development 124, 871–880 (1997)
Swarup, S. & Verheyen, E. M. Wnt/Wingless signaling in Drosophila. Cold Spring Harb. Perspect. Biol. 4, (2012)
Garcia-Bellido, A. & Merriam, J. R. Parameters of the wing imaginal disc development of Drosophila melanogaster. Dev. Biol. 24, 61–87 (1971)
Johnston, L. A. & Gallant, P. Control of growth and organ size in Drosophila. BioEssays 24, 54–64 (2002)
Martín, F. A., Herrera, S. C. & Morata, G. Cell competition, growth and size control in the Drosophila wing imaginal disc. Development 136, 3747–3756 (2009)
Williams, J. A., Paddock, S. W. & Carroll, S. B. Pattern formation in a secondary field: a hierarchy of regulatory genes subdivides the developing Drosophila wing disc into discrete subregions. Development 117, 571–584 (1993)
Couso, J. P., Knust, E. & Martinez Arias, A. Serrate and wingless cooperate to induce vestigial gene expression and wing formation in Drosophila. Curr. Biol. 5, 1437–1448 (1995)
Ng, M., Diaz-Benjumea, F. J., Vincent, J. P., Wu, J. & Cohen, S. M. Specification of the wing by localized expression of wingless protein. Nature 381, 316–318 (1996)
García-García, M. J., Ramain, P., Simpson, P. & Modolell, J. Different contributions of pannier and wingless to the patterning of the dorsal mesothorax of Drosophila. Development 126, 3523–3532 (1999)
Martinez Arias, A. Wnts as morphogens? The view from the wing of Drosophila. Nature Rev. Mol. Cell Biol. 4, 321–325 (2003)
Couso, J. P., Bishop, S. A. & Martinez Arias, A. The wingless signalling pathway and the patterning of the wing margin in Drosophila. Development 120, 621–636 (1994)
Nolo, R., Abbott, L. A. & Bellen, H. J. Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell 102, 349–362 (2000)
Jafar-Nejad, H., Tien, A.-C., Acar, M. & Bellen, H. J. Senseless and Daughterless confer neuronal identity to epithelial cells in the Drosophila wing margin. Development 133, 1683–1692 (2006)
Sato, A., Kojima, T., Ui-Tei, K., Miyata, Y. & Saigo, K. Dfrizzled-3, a new Drosophila Wnt receptor, acting as an attenuator of Wingless signaling in wingless hypomorphic mutants. Development 126, 4421–4430 (1999)
Sivasankaran, R., Calleja, M., Morata, G. & Basler, K. The Wingless target gene Dfz3 encodes a new member of the Drosophila Frizzled family. Mech. Dev. 91, 427–431 (2000)
Giraldez, A. J. & Cohen, S. M. Wingless and Notch signaling provide cell survival cues and control cell proliferation during wing development. Development 130, 6533–6543 (2003)
Baena-López, L. A., Franch-Marro, X. & Vincent, J.-P. Wingless promotes proliferative growth in a gradient-independent manner. Sci. Signal. 2, ra60 (2009)
Zecca, M. & Struhl, G. A feed-forward circuit linking wingless, fat-dachsous signaling, and the warts-hippo pathway to Drosophila wing growth. PLoS Biol. 8, e1000386 (2010)
Herr, P. & Basler, K. Porcupine-mediated lipidation is required for Wnt recognition by Wls. Dev. Biol. 361, 392–402 (2012)
Baena-López, L. A., Alexandre, C., Mitchell, A., Pashakarnis, L. & Vincent, J.-P. Accelerated genome engineering in Drosophila without sequence constraints. Development 140, 4818–4825 (2013)
Bergantiños, C., Corominas, M. & Serras, F. Cell death-induced regeneration in wing imaginal discs requires JNK signalling. Development 137, 1169–1179 (2010)
Piddini, E. & Vincent, J.-P. Interpretation of the wingless gradient requires signaling-induced self-inhibition. Cell 136, 296–307 (2009)
Dubois, L., Lecourtois, M., Alexandre, C., Hirst, E. & Vincent, J. P. Regulated endocytic routing modulates wingless signaling in Drosophila embryos. Cell 105, 613–624 (2001)
Lecuit, T. et al. Two distinct mechanisms for long-range patterning by Decapentaplegic in the Drosophila wing. Nature 381, 387–393 (1996)
Pérez, L. et al. Enhancer-PRE communication contributes to the expansion of gene expression domains in proliferating primordia. Development 138, 3125–3134 (2011)
Halder, G. et al. The Vestigial and Scalloped proteins act together to directly regulate wing-specific gene expression in Drosophila. Genes Dev. 12, 3900–3909 (1998)
Smith-Bolton, R. K., Worley, M. I., Kanda, H. & Hariharan, I. K. Regenerative growth in Drosophila imaginal discs is regulated by Wingless and Myc. Dev. Cell 16, 797–809 (2009)
Colombani, J., Andersen, D. S. & Léopold, P. Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science 336, 582–585 (2012)
Garelli, A., Gontijo, A. M., Miguela, V., Caparros, E. & Dominguez, M. Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science 336, 579–582 (2012)
Strigini, M. & Cohen, S. M. Wingless gradient formation in the Drosophila wing. Curr. Biol. 10, 293–300 (2000)
Giorgianni, M. W. & Mann, R. S. Establishment of medial fates along the proximodistal axis of the Drosophila leg through direct activation of dachshund by Distalless. Dev. Cell 20, 455–468 (2011)
Wilder, E. L. & Perrimon, N. Dual functions of wingless in the Drosophila leg imaginal disc. Development 121, 477–488 (1995)
Couso, J. P., Bate, M. & Martínez-Arias, A. A wingless-dependent polar coordinate system in Drosophila imaginal discs. Science 259, 484–489 (1993)
Artero, R., Furlong, E. E., Beckett, K., Scott, M. P. & Baylies, M. Notch and Ras signaling pathway effector genes expressed in fusion competent and founder cells during Drosophila myogenesis. Development 130, 6257–6272 (2003)
Alexandre, C., Lecourtois, M. & Vincent, J. Wingless and Hedgehog pattern Drosophila denticle belts by regulating the production of short-range signals. Development 126, 5689–5698 (1999)
Acknowledgements
This work was supported by the UK Medical Research Council (U117584268), an ERC grant (WNTEXPORT) from the European Union to JPV and a Sir Henry Wellcome post-doctoral fellowship to L.A.B-L. (082694/Z/07/Z). We are grateful to U.-M. Fiuza for discussion and A. Mitchell for help in generating the first wingless knockout allele. Discussions with G. Struhl have led to significant improvement of the manuscript. We thank colleagues listed in the Methods Summary, as well as the Developmental Studies Hybridoma Bank and the Bloomington Stock Center for providing antibodies and fly strains.
Author information
Authors and Affiliations
Contributions
All the experiments were performed jointly by L.A.B.-L. and C.A. C.A., L.A.B.-L., and J.-P.V. contributed equally to the conception of the work, the interpretation of results, and manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
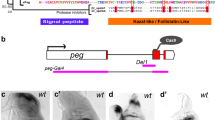
Extended Data Figure 1 Engineering the wg locus to express membrane-tethered Wg.
a, Structure of the wingless locus before targeting, after targeting, and after Cre-mediated excision. The wg{KO} allele was used as a founder line for subsequent reintegration. b, Cherry expression in wing, leg and haltere imaginal discs of larvae carrying one copy of wg{KO; Cherry}. c, Cuticle preparation of a homozygous wg{KO}larva at low and high magnification (black arrow). The phenotype is identical to that of wgCX4 homozygous embryos39. d, Diagram showing the reintegration of a wild type wingless cDNA in the wg{KO} to generate wg{KO; Wg} (note presence of mini-white). e, Diagram showing the reintegration of the NRT–Wg cDNA in wg{KO}. This was achieved using either pax-Cherry or mini-white as a genetic marker, as indicated. f, Wing of a wild-type fly. g, Wing of a wg{KO; Wg} homozygous fly. h, Overlay of the wings shown in Fig. 1b to illustrate the mild wing size reduction in NRT–Wg flies. i, Wing size of wg{KO; NRT–Wg} homozygous (n = 14) and control (wg{KO; NRT–Wg}/GlaBC) flies (n = 16, ***P < 0.001). j–l, High-magnification view of the wing margin of wild-type, homozygous wg{KO; Wg}, and homozygous wg{KO; NRT–Wg}. They are barely distinguishable. m–o, Views of the dorsal thorax illustrate the normal arrangement of pattern elements such as microchaetes and macrochaetes in the genotypes indicated. Error bars represent s.d. Statistical significance was assessed using Student’s t-test.
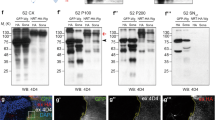
Extended Data Figure 2 Senseless expression and growth in wingless-null patches surrounded by wild-type or Neurotactin–Wingless-expressing cells.
a, b, Expression of Senseless (red) is lost in patches of wingless mutant cells (GFP-negative; wgCX4 homozygotes) except in the cells located within one cell diameter of surrounding GFP-positive cells, which are wild-type cells (a) or homozygous wg{KO; NRT–Wg} (b) (white arrows). Mosaics were created by mitotic recombination in a way that generates approximately the same number progenitors for the two genotypes, as described in Methods. c, Example of mosaic imaginal discs generated as above to measure the growth of wingless mutant territory (wgCX4 homozygous; GFP-negative) relative to that of wild type (c) or wg{KO; NRT–Wg} homozygous (d) tissue. Wg and NRT–Wg, detected with anti-Wg, are shown in red. e, Outline of the territory where the surface areas were assessed. f, Quantification of the areas colonised by wingless mutant cells (GFP-negative) in the two genetic backgrounds. On average, the wingless-null territory was smaller in the wg{KO; NRT–Wg} homozygous background (n = 24) than in the wild type (n = 20, ***P < 0.001). Error bars represent s.d. Statistical significance was assessed using Student’s t-test.
Extended Data Figure 3 Activity of the wingless promoter during imaginal disc development.
a, Timing of key developmental stages at 25 °C. b, Developmental timing at 18 °C as it relates to the results illustrated in c. c, Permanent labelling of wg-expressing cells and their descendants at different stages of development. Genotype was wg{KO; Gal4}, tubulin-gal80ts/UAS-Flp; Actin FRT stop FRT LacZ so that the stop cassette was only excised in cells that express wingless at the time of shifting to 29 °C to activate Flp expression and hence excision of the stop cassette. Discs were shifted from 18 °C to 29 °C at different stages (shown in b) but they were fixed and stained at the same stage, just before puparation.
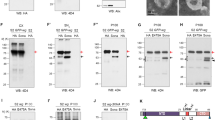
Extended Data Figure 4 Tissue-specific allele switching to determine the anatomical origin of organismal developmental delay in Neurotactin–Wingless animals.
a, Cumulative pattern of vestigial-gal4 activity in various organs precursors. Expression of vestigial-gal4 at any stage or place leads to excision of the stop cassette in Actin FRT stop FRT LacZ thus marking permanently the corresponding cells. As expected, nearly the whole wing and haltere discs were labelled at the end of larval development. In wing imaginal discs, only a few cells were β-Galactosidase-negative that did not overlap with the domain of Wg expression (anti-Wg, red). In the eye antennal disc, the patterns of Wg (white arrowhead) and β-Galactosidase expression are also non-overlapping. Therefore, in combination with UAS-Flp, vestigial-gal4 is expected to excise an FRT cassette throughout the domain of wingless expression. Examination of the brain and CNS shows that vestigial-gal4 is unexpectedly active in these tissues. b, In larvae of genotype vestigial-gal4, UAS-Flp, wg{FRT Wg FRT NRT–Wg}/Cyo wg, most of the wg-expressing cells in leg, haltere and wing imaginal discs, but not in the brain and CNS, were converted to expressing NRT-HA-Wg (anti-HA; green). c, Developmental timing in wg{KO; WTBody; NRT–WgDisc} (vestigial-gal4, UAS-Flp, wg{FRT Wg FRT NRT–Wg}) and control (vestigial-gal4, UAS-Flp, wg{FRT Wg FRT NRT–Wg}/GlaBC) larvae (80 animals, 4 experiments). The two data sets cannot be statistically distinguished (P > 0.05). d, Adult wing size for three genotypes: wg{KO; WTBody; NRT–WgDiscs}/GlaBc obtained from selfed vestigial-gal4, UAS-Flp, wg{FRT Wg FRT NRT–Wg}/GlaBc (n = 16, shown in black); wg{KO; WTBody; NRT–WgDiscs}, obtained from homozygous vestigial-gal4, UAS-Flp, wg{FRT Wg FRT NRT–Wg} (n = 15, shown in purple); and wg{KO; NRT–WgBody;WTDiscs}, obtained from homozygous vestigial-gal4, UAS-Flp, wg{FRT NRT–Wg FRT Wg} (n = 13, shown in grey). e–g, Extent of Distal-less expression in wg{KO; WTBody; NRT–WgDiscs} heterozygotes (over Cyo; e) and homozygotes (f). All the discs were obtained from immobile larvae at the time of anterior spiracle eversion, an event that marks the onset of pupariation. The extent of the Distal-less domain was estimated from the surface area of a polygon drawn around the zone of immunoreactivity, as shown. The results, plotted in panel g, show a mild reduction in wg{KO; WTBody; NRT–WgDiscs}discs (n = 13) compared to controls (n = 20; ***P < 0.001; n.s., not significantly different). Error bars represent s.d. Statistical significance was assessed using Student’s t-test.
Supplementary information
Supplementary Information
This file contains a Supplementary Note. (PDF 129 kb)
Rights and permissions
About this article
Cite this article
Alexandre, C., Baena-Lopez, A. & Vincent, JP. Patterning and growth control by membrane-tethered Wingless. Nature 505, 180–185 (2014). https://doi.org/10.1038/nature12879
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12879
This article is cited by
-
The USP46 deubiquitylase complex increases Wingless/Wnt signaling strength by stabilizing Arrow/LRP6
Nature Communications (2023)
-
Nodal is a short-range morphogen with activity that spreads through a relay mechanism in human gastruloids
Nature Communications (2022)
-
GPI-anchored FGF directs cytoneme-mediated bidirectional contacts to regulate its tissue-specific dispersion
Nature Communications (2022)
-
Cell fate specification and differentiation in the adult mammalian intestine
Nature Reviews Molecular Cell Biology (2021)
-
Domestication of chemicals attacking metazoan embryogenesis: identification of safe natural products modifying developmental signaling pathways in human
The Journal of Antibiotics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.