Abstract
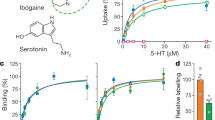
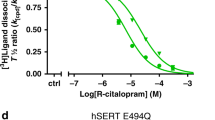
The biogenic amine transporters (BATs) regulate endogenous neurotransmitter concentrations and are targets for a broad range of therapeutic agents including selective serotonin reuptake inhibitors (SSRIs), serotonin–noradrenaline reuptake inhibitors (SNRIs) and tricyclic antidepressants (TCAs)1,2. Because eukaryotic BATs are recalcitrant to crystallographic analysis, our understanding of the mechanism of these inhibitors and antidepressants is limited. LeuT is a bacterial homologue of BATs and has proven to be a valuable paradigm for understanding relationships between their structure and function3. However, because only approximately 25% of the amino acid sequence of LeuT is in common with that of BATs, and as LeuT is a promiscuous amino acid transporter4, it does not recapitulate the pharmacological properties of BATs. Indeed, SSRIs and TCAs bind in the extracellular vestibule of LeuT5,6,7 and act as non-competitive inhibitors of transport5. By contrast, multiple studies demonstrate that both TCAs and SSRIs are competitive inhibitors for eukaryotic BATs and bind to the primary binding pocket8,9,10,11,12,13,14,15,16. Here we engineered LeuT to harbour human BAT-like pharmacology by mutating key residues around the primary binding pocket. The final LeuBAT mutant binds the SSRI sertraline with a binding constant of 18 nM and displays high-affinity binding to a range of SSRIs, SNRIs and a TCA. We determined 12 crystal structures of LeuBAT in complex with four classes of antidepressants. The chemically diverse inhibitors have a remarkably similar mode of binding in which they straddle transmembrane helix (TM) 3, wedge between TM3/TM8 and TM1/TM6, and lock the transporter in a sodium- and chloride-bound outward-facing open conformation. Together, these studies define common and simple principles for the action of SSRIs, SNRIs and TCAs on BATs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bröer, S. & Gether, U. The solute carrier 6 family of transporters. Br. J. Pharmacol. 167, 256–278 (2012)
Kristensen, A. S. et al. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol. Rev. 63, 585–640 (2011)
Yamashita, A., Singh, S. K., Kawate, T., Jin, Y. & Gouaux, E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature 437, 215–223 (2005)
Singh, S. K., Piscitelli, C. L., Yamashita, A. & Gouaux, E. A competitive inhibitor traps LeuT in an open-to-out conformation. Science 322, 1655–1661 (2008)
Singh, S. K., Yamashita, A. & Gouaux, E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature 448, 952–956 (2007)
Zhou, Z. et al. LeuT-desipramine structure reveals how antidepressants block neurotransmitter reuptake. Science 317, 1390–1393 (2007)
Zhou, Z. et al. Antidepressant specificity of serotonin transporter suggested by three LeuT–SSRI structures. Nature Struct. Mol. Biol. 16, 652–657 (2009)
Talvenheimo, J., Nelson, P. J. & Rudnick, G. Mechanism of imipramine inhibition of platelet 5-hydroxytryptamine transport. J. Biol. Chem. 254, 4631–4635 (1979)
Barker, E. L. et al. High affinity recognition of serotonin transporter antagonists defined by species-scanning mutagenesis: an aromatic residue in transmembrane domain I dictates species-selective recognition of citalopram and mazindol. J. Biol. Chem. 273, 19459–19468 (1998)
Henry, L. K. et al. Tyr-95 and Ile-172 in transmembrane segments 1 and 3 of human serotonin transporters interact to establish high affinity recognition of antidepressants. J. Biol. Chem. 281, 2012–2023 (2006)
Andersen, J. et al. Location of the antidepressant binding site in the serotonin transporter: importance of Ser-438 in recognition of citalopram and tricyclic antidepressants. J. Biol. Chem. 284, 10276–10284 (2009)
Sinning, S. et al. Binding and orientation of tricyclic antidepressants within the central substrate site of the human serotonin transporter. J. Biol. Chem. 285, 8363–8374 (2010)
Tavoulari, S., Forrest, L. R. & Rudnick, G. Fluoxetine (Prozac) binding to serotonin transporter is modulated by chloride and conformational changes. J. Neurosci. 29, 9635–9643 (2009)
Koldsø, H. et al. The two enantiomers of citalopram bind to the human serotonin transporter in reversed orientations. J. Am. Chem. Soc. 132, 1311–1322 (2010)
Andersen, J. et al. Mutational mapping and modeling of the binding site for (S)-Citalopram in the human serotonin transporter. J. Biol. Chem. 285, 2051–2063 (2010)
Jørgensen, A. M. et al. Homology modeling of the serotonin transporter: insights into the primary escitalopram-binding site. ChemMedChem 2, 815–826 (2007)
Forrest, L. R. & Rudnick, G. The rocking bundle: a mechanism for ion-coupled solute flux by symmetrical transporters. Physiology 24, 377–386 (2009)
Zomot, E. et al. Mechanism of chloride interaction with neurotransmitter:sodium symporters. Nature 449, 726–730 (2007)
Forrest, L. R., Tavoulari, S., Zhang, Y.-W., Rudnick, G. & Honig, B. Identification of a chloride ion binding site in Na+/Cl− dependent transporters. Proc. Natl Acad. Sci. USA 104, 12761–12766 (2007)
Celik, L. et al. Binding of serotonin to the human serotonin transporter. Molecular modeling and experimental validation. J. Am. Chem. Soc. 130, 3853–3865 (2008)
Kaufmann, K. W. et al. Structural determinants of species-selective substrate recognition in human and Drosophila serotonin transporters revealed through computational docking studies. Proteins 74, 630–642 (2009)
Tatsumi, M., Groshan, K., Blakely, R. D. & Richelson, E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur. J. Pharmacol. 340, 249–258 (1997)
Sarker, S. et al. The high-affinity binding site for tricyclic antidepressants resides in the outer vestibule of the serotonin transporter. Mol. Pharmacol. 78, 1026–1035 (2010)
Tavoulari, S., Rizwan, A. N., Forrest, L. R. & Rudnick, G. Reconstructing a chloride-binding site in a bacterial neurotransmitter transporter homologue. J. Biol. Chem. 286, 2834–2842 (2011)
Wang, H., Elferich, J. & Gouaux, E. Structures of LeuT in bicelles define conformation and substrate binding in a membrane-like context. Nature Struct. Mol. Biol. 19, 212–219 (2012)
Quick, M. et al. Binding of an octylglucoside detergent molecule in the second substrate (S2) site of LeuT establishes an inhibitor-bound conformation. Proc. Natl Acad. Sci. USA 106, 5563–5568 (2009)
Sørensen, L. et al. Interaction of antidepressants with the serotonin and norepinephrine transporters: mutational studies of the S1 substrate binding pocket. J. Biol. Chem. 287, 43694–43707 (2012)
Andersen, J. et al. Molecular determinants for selective recognition of antidepressants in the human serotonin and norepinephrine transporters. Proc. Natl Acad. Sci. USA 108, 12137–12142 (2011)
Penmatsa, A., Wang, K. H. & Gouaux, E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature http://dx.doi.org/10.1038/nature12533 (15 September 2013)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Battye, T. G. G., Kontogiannis, L., Johnson, O., Powell, H. R. & Leslie, A. G. W. iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr. D 67, 271–281 (2011)
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007)
Collaborative Computing Project. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010)
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010)
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010)
Wang, H. & Gouaux, E. Substrate binds in the S1 site of the F253A mutant of LeuT, a neurotransmitter sodium symporter homologue. EMBO Rep. 13, 861–866 (2012)
Kawate, T. & Gouaux, E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure 14, 673–681 (2006)
Krishnamurthy, H. & Gouaux, E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature 481, 469–474 (2012)
Acknowledgements
We thank J. Michel for help in binding experiments, D. Claxton for comments and L. Vaskalis for assistance with illustrations. We also thank the beamline staff at the Advanced Light Source (beamlines 8.2.1 and 5.0.2) and Advanced Photon Source (Argonne National Laboratory, beamlines 24-ID-C and 24-ID-E). H.W. also thanks the presenters at 2012 CCP4/APS summer school for useful lectures and tutorials. This work was supported by the National Institutes of Health. E.G. is an Investigator with the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
H.W. and E.G. designed the research; H.W., A.G. and R.R. performed protein expression and purification; H.W., A.G., K.H.W. and A.P. carried out ligand-binding and flux experiments; H.W. conducted crystallization and structure determination; H.W. and E.G. wrote the manuscript together with comments from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Coordinates and structure factors for the LeuBAT Δ13-paroxetine, Δ13-sertraline, Δ13-duloxetine, Δ13-desvenlafaxine, Δ13-fluoxetine, Δ13-fluvoxamine, Δ13-clomipramine, Δ6-sertraline, Δ6-desvenlafaxine, Δ6-duloxetine, Δ6-mazindol and Δ5-mazindol crystal structures have been deposited in the Protein Data Bank with codes 4MM4, 4MM5, 4MM6, 4MM7, 4MM8, 4MM9, 4MMA, 4MMB, 4MMC, 4MMD, 4MME and 4MMF, respectively.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-10 and Supplementary Tables 1-4. (PDF 1782 kb)
Rights and permissions
About this article
Cite this article
Wang, H., Goehring, A., Wang, K. et al. Structural basis for action by diverse antidepressants on biogenic amine transporters. Nature 503, 141–145 (2013). https://doi.org/10.1038/nature12648
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12648
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.