Abstract
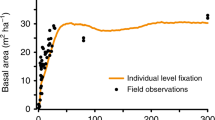
Forests contribute a significant portion of the land carbon sink, but their ability to sequester CO2 may be constrained by nitrogen1,2,3,4,5,6, a major plant-limiting nutrient. Many tropical forests possess tree species capable of fixing atmospheric dinitrogen (N2)7, but it is unclear whether this functional group can supply the nitrogen needed as forests recover from disturbance or previous land use1, or expand in response to rising CO2 (refs 6, 8). Here we identify a powerful feedback mechanism in which N2 fixation can overcome ecosystem-scale deficiencies in nitrogen that emerge during periods of rapid biomass accumulation in tropical forests. Over a 300-year chronosequence in Panama, N2-fixing tree species accumulated carbon up to nine times faster per individual than their non-fixing neighbours (greatest difference in youngest forests), and showed species-specific differences in the amount and timing of fixation. As a result of fast growth and high fixation, fixers provided a large fraction of the nitrogen needed to support net forest growth (50,000 kg carbon per hectare) in the first 12 years. A key element of ecosystem functional diversity was ensured by the presence of different N2-fixing tree species across the entire forest age sequence. These findings show that symbiotic N2 fixation can have a central role in nitrogen cycling during tropical forest stand development, with potentially important implications for the ability of tropical forests to sequester CO2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Davidson, E. A. et al. Recuperation of nitrogen cycling in Amazonian forests following agricultural abandonment. Nature 447, 995–998 (2007)
Zaehle, S. & Dalmonech, D. Carbon–nitrogen interactions on land at global scales: Current understanding in modeling climate biosphere feedbacks. Curr. Opin. Environ. Sustain. 3, 311–320 (2011)
Gerber, S., Hedin, L. O., Oppenheimer, M., Pacala, S. W. & Shevliakova, E. Nitrogen cycling and feedbacks in a global dynamic land model. Glob. Biogeochem. Cycles 24, GB1001 (2010)
Thornton, P. E., Lamarque, J.-F., Rosenbloom, N. A. & Mahowald, N. M. Influence of carbon–nitrogen cycle coupling on land model response to CO2 fertilization and climate variability. Glob. Biogeochem. Cycles 21, GB4108 (2007)
Houghton, R. A., Hall, F. & Goetz, S. J. Importance of biomass in the global carbon cycle. J. Geophys. Res. 114, G00E03 (2009)
Hungate, B. A., Dukes, J. S., Shaw, R., Luo, Y. & Field, C. Nitrogen and climate change. Science 302, 1512–1513 (2003)
Sprent, J. I. Legume Nodulation: A Global Perspective. (Wiley-Blackwell, 2009)
Goll, D. S. et al. Nutrient limitation reduces land carbon uptake in simulations with a model of combined carbon, nitrogen and phosphorus cycling. Biogeosci. Discuss. 9, 3173–3232 (2012)
Brown, S. & Lugo, A. E. Tropical secondary forests. J. Trop. Ecol. 6, 1–32 (1990)
Pan, Y. et al. A large and persistent carbon sink in the world’s forests. Science 333, 988–993 (2011)
Davidson, E. A. et al. Nitrogen and phosphorus limitation of biomass growth in a tropical secondary forest. Ecol. Appl. 14, S150–S163 (2004)
Amazonas, N. T., Martinelli, L. A., Piccolo, M. C. & Rodrigues, R. R. Nitrogen dynamics during ecosystem development in tropical forest restoration. For. Ecol. Manage. 262, 1551–1557 (2011)
Russell, A. E. & Raich, J. W. Rapidly growing tropical trees mobilize remarkable amounts of nitrogen, in ways that differ surprisingly among species. Proc. Natl Acad. Sci. USA 109, 10398–10402 (2012)
Gehring, C., Vlek, P. L. G., de Souza, L. A. G. & Denich, M. Biological nitrogen fixation in secondary regrowth and mature rainforest of central Amazonia. Agric. Ecosyst. Environ. 111, 237–252 (2005)
Barron, A. R., Purves, D. W. & Hedin, L. O. Facultative nitrogen fixation by canopy legumes in a lowland tropical forest. Oecologia 165, 511–520 (2011)
Binkley, D., Senock, R. & Cromack, K., Jr Phosphorus limitation on nitrogen fixation by Facaltaria seedlings. For. Ecol. Manage. 186, 171–176 (2003)
Vitousek, P. M. & Howarth, R. W. Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13, 87–115 (1991)
van Groenigen, K. J. et al. Element interactions limit soil carbon storage. Proc. Natl Acad. Sci. USA 103, 6571–6574 (2006)
Hedin, L. O., Brookshire, E. N. J., Menge, D. N. L. & Barron, A. R. The nitrogen paradox in tropical forest ecosystems. Annu. Rev. Ecol. Evol. Syst. 40, 613–635 (2009)
Isbell, F. et al. High plant diversity is needed to maintain ecosystem services. Nature 477, 199–202 (2011)
Yang, X., Richardson, T. K. & Jain, A. K. Contributions of secondary forest and nitrogen dynamics to terrestrial carbon uptake. Biogeosciences 7, 3041–3050 (2010)
van Breugel, M., Ransijn, J., Craven, D., Bongers, F. & Hall, J. S. Estimating carbon stock in secondary forests: decisions and uncertainties associated with allometric biomass models. For. Ecol. Manage. 262, 1648–1657 (2011)
Quesada, C. A. et al. Variations in chemical and physical properties of Amazon forest soils in relation to their genesis. Biogeosciences 7, 1515–1541 (2010)
Fyllas, N. M. et al. Basin-wide variations in foliar properties of Amazonian forest: phylogeny, soils and climate. Biogeosci. Discuss. 6, 3707–3769 (2009)
Chave, J. et al. Error propagation and scaling for tropical forest biomass estimates. Phil. Trans. R. Soc. Lond. B 359, 409–420 (2004)
Menge, D. N. & Hedin, L. O. Nitrogen fixation in different biogeochemical niches along a 120000-year chronosequence in New Zealand. Ecology 90, 2190–2201 (2009)
Batterman, S. A., Wurzburger, N. & Hedin, L. O. Nitrogen and phosphorus interact to control tropical symbiotic N2 fixation: a test in Inga punctata. J. Ecology (in the press)
Barron, A. R. et al. Molybdenum limitation of asymbiotic nitrogen fixation in tropical forest soils. Nature Geosci. 2, 42–45 (2009)
Hassler, S. K., Zimmerman, B., van Breugel, M., Hall, J. S. & Elsenbeer, H. Recovery of saturated hydraulic conductivity under secondary succession on former pasture in the humid tropics. For. Ecol. Manage. 261, 1634–1642 (2011)
Neumann-Cosel, L., Zimmerman, B., Hall, J. S., van Breugel, M. & Elsenbeer, H. Soil carbon dynamics under young tropical secondary forests on former pastures—a case study from Panama. For. Ecol. Manage. 261, 1625–1633 (2011)
Walker, L. R., Wardle, D. A., Bardgett, R. D. & Clarkson, B. D. The use of chronosequences in studies of ecological succession and soil development. J. Ecol. 98, 725–736 (2010)
Jackson, R. B., Mooney, H. A. & Schulze, E. D. A global budget for fine root biomass, surface area, and nutrient contents. Proc. Natl Acad. Sci. USA 94, 7362–7366 (1997)
Wolf, A., Field, C. & Berry, J. A. Allometric growth and allocation in forests: a perspective from FLUXNET. Ecol. Appl. 21, 1546–1556 (2011)
Martin, A. R. & Thomas, S. C. A reassessment of carbon content in tropical trees. PLoS ONE 6, e23533 (2011)
Craven, D. J. Dynamics of Tropical Secondary Forests in Central Panama: Linking Functional Traits with Ecological Performance during Succession. PhD thesis, Yale Univ. (2012)
Townsend, A. R., Cleveland, C. C., Asner, G. P. & Bustamente, M. M. C. Controls over foliar N:P ratios in tropical rain forests. Ecology 88, 107–118 (2007)
Martius, C. Density, humidity, and nitrogen content of dominant wood species of floodplain forests (várzea) in Amazonia. Eur. J. Wood Wood Products 50, 300–303 (1992)
Chave, J. et al. Towards a worldwide wood economics spectrum. Ecol. Lett. 12, 351–366 (2009)
Muller-Landau, H. C. et al. Testing metabolic ecology theory for allometric scaling of tree size, growth and mortality in tropical forests. Ecol. Lett. 9, 575–588 (2006)
Barron, A. R. Patterns and Controls of Nitrogen Fixation in a Lowland Tropical Forest, Panama. PhD thesis, Princeton Univ. (2007)
Acknowledgements
We thank S. Adelberg and K. Zelazny for assisting with data collection, M. Baillon and A. Hernandez for botanical identifications, N. Wurzburger and A. Barron for species-specific N2 fixation rates, J. Sprent for advice about N2-fixing trees and P. Reich for comments. This work was supported by grants to L.O.H. from the National Science Foundation (NSF; DEB-0614116), the National Oceanic and Atmospheric Association (NOAA; grant NA17RJ262 – 344), the Cooperative Institute for Climate Science of Princeton University and the Carbon Mitigation Initiative of Princeton University; and to S.A.B. from the Smithsonian Tropical Research Institute (STRI). It is a contribution to the Agua Salud Project (ASP), a collaboration among STRI, the Panama Canal Authority (ACP) and the National Environmental Authority of Panama (ANAM). ASP funding came from the HSBC climate partnership, STRI, the Frank Levinson Family Foundation, the Motta Family Foundation and an anonymous donor.
Author information
Authors and Affiliations
Contributions
S.A.B., L.O.H., J.S.H. and M.v.B. designed the project. S.A.B. conducted N2-fixation-related field work. J.R., M.v.B. and J.S.H. provided allometry data; D.J.C. provided plant nutrient data. S.A.B. and L.O.H. wrote the paper. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Notes 1-8, Supplementary Tables 1-7, Supplementary Figures 1-7 and Supplementary References. (PDF 1970 kb)
Supplementary Information
This file contains the Plant-soil N dynamics model, which should be run in code R1. (PDF 129 kb)
PowerPoint slides
Rights and permissions
About this article
Cite this article
Batterman, S., Hedin, L., van Breugel, M. et al. Key role of symbiotic dinitrogen fixation in tropical forest secondary succession. Nature 502, 224–227 (2013). https://doi.org/10.1038/nature12525
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12525
This article is cited by
-
Biological nitrogen fixation in young and old tropical forests under five contrasting edaphoclimatic conditions
Nutrient Cycling in Agroecosystems (2024)
-
Can we see the nitrate from the trees? Long-term linkages between tropical forest productivity and stream nitrogen concentrations
Biogeochemistry (2023)
-
Quantifying the Effect Size of Management Actions on Aboveground Carbon Stocks in Forest Plantations
Current Forestry Reports (2023)
-
Interaction of nitrogen availability in the soil with leaf physiological traits and nodule formation of Robinia pseudoacacia-rhizobia symbiosis depends on provenance
Plant and Soil (2023)
-
Increasing reclamation ages drive shifts in carbon and nitrogen stoichiometry and natural isotopes from leaf-litter-root-soil continuum in a reclamation ecosystem, North China
Journal of Soils and Sediments (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.