Abstract
Bacillus anthracis, the causative agent of anthrax disease, is lethal owing to the actions of two exotoxins: anthrax lethal toxin (LT) and oedema toxin (ET). The key tissue targets responsible for the lethal effects of these toxins are unknown. Here we generated cell-type-specific anthrax toxin receptor capillary morphogenesis protein-2 (CMG2)-null mice and cell-type-specific CMG2-expressing mice and challenged them with the toxins. Our results show that lethality induced by LT and ET occurs through damage to distinct cell types; whereas targeting cardiomyocytes and vascular smooth muscle cells is required for LT-induced mortality, ET-induced lethality occurs mainly through its action in hepatocytes. Notably, and in contradiction to what has been previously postulated, targeting of endothelial cells by either toxin does not seem to contribute significantly to lethality. Our findings demonstrate that B. anthracis has evolved to use LT and ET to induce host lethality by coordinately damaging two distinct vital systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Moayeri, M. & Leppla, S. H. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol. Aspects Med. 30, 439–455 (2009)
Bradley, K. A., Mogridge, J., Mourez, M., Collier, R. J. & Young, J. A. Identification of the cellular receptor for anthrax toxin. Nature 414, 225–229 (2001)
Scobie, H. M., Rainey, G. J., Bradley, K. A. & Young, J. A. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc. Natl Acad. Sci. USA 100, 5170–5174 (2003)
Liu, S. et al. Capillary morphogenesis protein-2 is the major receptor mediating lethality of anthrax toxin in vivo. Proc. Natl Acad. Sci. USA 106, 12424–12429 (2009)
Leppla, S. H. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl Acad. Sci. USA 79, 3162–3166 (1982)
Firoved, A. M. et al. Bacillus anthracis edema toxin causes extensive tissue lesions and rapid lethality in mice. Am. J. Pathol. 167, 1309–1320 (2005)
Duesbery, N. S. et al. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280, 734–737 (1998)
Vitale, G. et al. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem. Biophys. Res. Commun. 248, 706–711 (1998)
Vitale, G., Bernardi, L., Napolitani, G., Mock, M. & Montecucco, C. Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem. J. 352, 739–745 (2000)
Newman, Z. L. et al. Susceptibility to anthrax lethal toxin-induced rat death is controlled by a single chromosome 10 locus that includes rNlrp1. PLoS Pathog. 6, e1000906 (2010)
Levinsohn, J. L. et al. Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS Pathog. 8, e1002638 (2012)
Pezard, C., Berche, P. & Mock, M. Contribution of individual toxin components to virulence of Bacillus anthracis. Infect. Immun. 59, 3472–3477 (1991)
Liu, S. et al. Anthrax toxin targeting of myeloid cells through the CMG2 receptor is essential for establishment of Bacillus anthracis infections in mice. Cell Host Microbe 8, 455–462 (2010)
Guichard, A., Nizet, V. & Bier, E. New insights into the biological effects of anthrax toxins: linking cellular to organismal responses. Microbes Infect. 5, 48–61 (2012)
Moayeri, M., Haines, D., Young, H. A. & Leppla, S. H. Bacillus anthracis lethal toxin induces TNF-independent hypoxia-mediated toxicity in mice. J. Clin. Invest. 112, 670–682 (2003)
Moayeri, M. et al. The heart is an early target of anthrax lethal toxin in mice: a protective role for neuronal nitric oxide synthase (nNOS). PLoS Pathog. 5, e1000456 (2009)
Bolcome, R. E., III et al. Anthrax lethal toxin induces cell death-independent permeability in zebrafish vasculature. Proc. Natl Acad. Sci. USA 105, 2439–2444 (2008)
Guichard, A. et al. Anthrax toxins cooperatively inhibit endocytic recycling by the Rab11/Sec15 exocyst. Nature 467, 854–858 (2010)
Warfel, J. M., Steele, A. D. & D’Agnillo, F. Anthrax lethal toxin induces endothelial barrier dysfunction. Am. J. Pathol. 166, 1871–1881 (2005)
Maddugoda, M. P. et al. cAMP signaling by anthrax edema toxin induces transendothelial cell tunnels, which are resealed by MIM via Arp2/3-driven actin polymerization. Cell Host Microbe 10, 464–474 (2011)
Ghosh, C. C. et al. Impaired function of the Tie-2 receptor contributes to vascular leakage and lethality in anthrax. Proc. Natl Acad. Sci. USA 109, 10024–10029 (2012)
Arora, N., Klimpel, K. R., Singh, Y. & Leppla, S. H. Fusions of anthrax toxin lethal factor to the ADP-ribosylation domain of Pseudomonas exotoxin A are potent cytotoxins which are translocated to the cytosol of mammalian cells. J. Biol. Chem. 267, 15542–15548 (1992)
Liu, S. et al. Diphthamide modification on eukaryotic elongation factor 2 is needed to assure fidelity of mRNA translation and mouse development. Proc. Natl Acad. Sci. USA 109, 13817–13822 (2012)
Bradley, S. V. et al. Degenerative phenotypes caused by the combined deficiency of murine HIP1 and HIP1r are rescued by human HIP1. Hum. Mol. Genet. 16, 1279–1292 (2007)
Lepore, J. J. et al. High-efficiency somatic mutagenesis in smooth muscle cells and cardiac myocytes in SM22α-Cre transgenic mice. Genesis 41, 179–184 (2005)
Dal Molin, F. et al. Ratio of lethal and edema factors in rabbit systemic anthrax. Toxicon 52, 824–828 (2008)
Sirard, J. C., Mock, M. & Fouet, A. The three Bacillus anthracis toxin genes are coordinately regulated by bicarbonate and temperature. J. Bacteriol. 176, 5188–5192 (1994)
Mabry, R. et al. Detection of anthrax toxin in the serum of animals infected with Bacillus anthracis by using engineered immunoassays. Clin. Vaccine Immunol. 13, 671–677 (2006)
Jernigan, J. A. et al. Bioterrorism-related inhalational anthrax: The first 10 cases reported in the United States. Emerg. Infect. Dis. 7, 933–944 (2001)
Guarner, J. et al. Pathology and pathogenesis of bioterrorism-related inhalational anthrax. Am. J. Pathol. 163, 701–709 (2003)
Pomerantsev, A. P., Sitaraman, R., Galloway, C. R., Kivovich, V. & Leppla, S. H. Genome engineering in Bacillus anthracis using Cre recombinase. Infect. Immun. 74, 682–693 (2006)
Hu, H., Sa, Q., Koehler, T. M., Aronson, A. I. & Zhou, D. Inactivation of Bacillus anthracis spores in murine primary macrophages. Cell. Microbiol. 8, 1634–1642 (2006)
Reynolds, L. E. & Hodivala-Dilke, K. M. Primary mouse endothelial cell culture for assays of angiogenesis. Methods Mol. Med. 120, 503–509 (2006)
Liu, S. & Leppla, S. H. Cell surface tumor endothelium marker 8 cytoplasmic tail-independent anthrax toxin binding, proteolytic processing, oligomer formation, and internalization. J. Biol. Chem. 278, 5227–5234 (2003)
Alva, J. A. et al. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev. Dyn. 235, 759–767 (2006)
Agah, R. et al. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J. Clin. Invest. 100, 169–179 (1997)
Holtwick, R. et al. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc. Natl Acad. Sci. USA 99, 7142–7147 (2002)
Postic, C. et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J. Biol. Chem. 274, 305–315 (1999)
Braunstein, E. M. et al. Villin: A marker for development of the epithelial pyloric border. Dev. Dyn. 224, 90–102 (2002)
Hall, B. E. et al. Conditional overexpression of TGF-β1 disrupts mouse salivary gland development and function. Lab. Invest. 90, 543–555 (2010)
Pomerantsev, A. P. et al. A Bacillus anthracis strain deleted for six proteases serves as an effective host for production of recombinant proteins. Protein Expr. Purif. 80, 80–90 (2011)
Liu, S., Leung, H. J. & Leppla, S. H. Characterization of the interaction between anthrax toxin and its cellular receptors. Cell. Microbiol. 9, 977–987 (2007)
Gupta, P. K., Moayeri, M., Crown, D., Fattah, R. J. & Leppla, S. H. Role of N-terminal amino acids in the potency of anthrax lethal factor. PLoS ONE 3, e3130 (2008)
Rosovitz, M. J. et al. Alanine scanning mutations in domain 4 of anthrax toxin protective antigen reveal residues important for binding to the cellular receptor and to a neutralizing monoclonal antibody. J. Biol. Chem. 278, 30936–30944 (2003)
Acknowledgements
This research was supported by the intramural research programs of the National Institute of Allergy and Infectious Diseases and the National Heart, Lung, and Blood Institute, National Institutes of Health. We thank L. Feigenbaum and the staff at SAIC/NCI Frederick for generation of the founder CMG2 transgenic mice. We thank A. Kulkarni, B. Hall, B. Klaunberg, S. Anderson, I. Sastalla, C. Leysath and C. Bachran for discussions, and D. Despres for help with echocardiography.
Author information
Authors and Affiliations
Contributions
Y.Z. maintained mouse colonies and performed animal experiments. M.M. and J.L. designed, performed experiments, analysed data and edited the paper. D.C. performed animal experiments. R.J.F. purified proteins. A.N.W. made the CMG2 transgenic construct. Z.-X.Y. performed histological analyses. T.F. was involved in scientific discussions, providing reagents, and edited the paper. S.H.L. supervised research and edited the paper. S.L. conceived and supervised the project, designed and performed experiments, analysed data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Generation of endothelial-cell-specific CMG2-null mice.
a, Strategy for generation of endothelial-cell-specific CMG2-null mice. Diagram shows CMG2fl allele having exon 12 (encoding transmembrane domain, TM) flanked by loxP sites and the endothelial-cell-specific CMG2-null allele (CMG2(EC)−). The red arrowheads indicate loxP sites. The homozygous endothelial-cell-specific CMG2-null mice (CMG2(EC)−/−) were obtained by intercrossing of CMG2(EC)+/−mice. Other cell-type-specific CMG2-null mice were made similarly by using the corresponding cell-type-specific Cre transgenic mice. b, RT–PCR analyses of CMG2 transmembrane (TM) domain deletion in various tissues of CMG2(EC)−/− mice. Primers flanking the CMG2 transmembrane domain were used to amplify a CMG2 cDNA fragment. Endothelial cells and non-endothelial cells were isolated simultaneously from lungs pooled from three CMG2(EC)−/− mice and three CMG2fl/fl control mice. Representative of two independent experiments is shown. Expression of TEM8 and GAPDH in these samples is also shown. c, Sensitivity of endothelial cells and non-endothelial cells from CMG2fl/fl and CMG2(EC)−/− mice to PA plus FP59. Cells were treated with various concentrations of PA and FP59 (100 ng ml−1) for 48 h. Cell viability was evaluated by MTT assay, expressed as relative MTT signals to untreated cells. Error bars indicate s.d. d, Resistance of endothelial cells from CMG2(EC)−/− mice to LT. Endothelial cells from CMG2(EC)−/− and wild-type mice were treated with various concentrations of LF and PA (500 ng ml−1) for 48 h. e, PA(L687A) preferentially kills CMG2-expressing cells. Cells were treated with various concentrations of PA or PA(L687A) and 100 ng ml−1 FP59 for 48 h. PR230(TEM8) and PR230(CMG2) are engineered CHO cells expressing only TEM8 or CMG2. Note that PR230(TEM8) cells are 100-fold more resistant than PR230(CMG2) cells to PA(L687A) plus FP59. f, Sensitivity of endothelial cells and non-endothelial cells from CMG2fl/fl and CMG2(EC)−/− mice to PA(L687A) plus FP59. Cells were incubated for 48 h with various concentrations of PA(L687A) and 100 ng ml−1 FP59. Error bars indicate s.d. g, Susceptibility of CMG2(EC)−/− mice to LT. CMG2(EC)−/− mice and their littermate controls were injected intravenously with 50 μg LT (50 μg PA plus 50 μg LF), and monitored for survival. Whole-body CMG2−/− mice were included as additional controls. h, Disease progression of the LT-challenged mice in panel g. Please see Methods for disease progression scoring criteria.
Extended Data Figure 2 Generation of endothelial-cell-specific CMG2-expressing mice.
a, Strategy for generation of endothelial-cell-specific CMG2-expressing mice. In the CMG2 transgenic vector (LSL-CMG2), a loxP-stop-loxP cassette containing a promoterless eGFP and a poly(A) stop signal flanked by loxP sites was placed between the CAG promoter and CMG2 cDNA. Activation of CMG2 transgene in endothelial cells (CMG2EC) was achieved by breeding LSL-CMG2 mice with Cdh-cre mice to specifically remove the loxP-stop-loxP cassette in endothelial cells. Other cell-type-specific CMG2-expressing mice were made similarly by using the corresponding cell-type-specific Cre transgenic mice. b, c, Regained toxin sensitivity of endothelial cells from CMG2EC mice. Endothelial cells and non-endothelial cells from CMG2EC and whole-body CMG2−/− mice were incubated for 48 h with various concentrations of PA (b) or PA(L687A) (c) and 100 ng ml−1 FP59. Error bars indicate s.d.
Extended Data Figure 3 Tissue-specific deletion of CMG2 in CMG2(CM)−/− and CMG2(SM/CM)−/− mice.
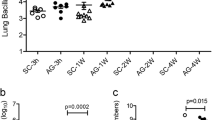
a, RT–PCR analyses of CMG2 deletion in tissues of CMG2(CM )−/− and CMG2(SM/CM)−/− mice. CMG2 deletion was detected in the hearts of CMG2(CM)−/− mice and in the hearts and aorta of CMG2(SM/CM)−/− mice. The small fraction of CMG2 deletion that occurred in other tissues of the CMG2(SM/CM)−/− mice was due to the existence of varying amounts of vascular smooth muscle cells in those tissues. Representative of two independent experiments is shown. b, Resistance of smooth-muscle/cardiomyocyte-specific CMG2-null mice to LT. CMG2(SM/CM)−/− mice and their littermate CMG2(SM/CM)+/− controls were injected intravenously with 50 μg LT, and monitored for survival. Whole-body CMG2−/− mice were included as additional controls. Right panel shows the disease progression of the challenged mice. CMG2(SM/CM)−/− versus CMG2+/+ mice, P = 0.0002. Log-rank test.
Extended Data Figure 4 Fluorescence microscopic analyses of GFP expression in mouse tissues.
a, Representative fluorescence microscopy of skeletal muscle (1), aorta (vascular smooth muscle) (2), small intestine (smooth muscle) (3), liver (4), lung (5), spleen (6) and kidney (cortex) (7) from CMG2CM mice (n = 2). Scale bar, 100 μm. b, Representative fluorescence microscopy of skeletal muscle (1), liver (2), kidney (cortex) (3), spleen (4) and uterus (5) from CMG2SM/CM mice (n = 3), and uterus (6) from LSL-CMG2 mice (n = 2). Scale bar, 100 μm.
Extended Data Figure 5 Histology of heart and liver of LT- and ET-treated mice.
a, Haematoxylin and eosin staining of heart and liver from wild-type (n = 3) and CMG2−/− (n = 3) mice challenged intraperitoneally with 100 μg LT for 48 h. In heart, regions with cardiomyocyte degeneration were found in LT-treated wild-type but not CMG2−/− mice. Arrows show examples of degenerated cardiomyocytes. In liver, regions with mild to modest hepatocyte degeneration were identified in LT-treated wild-type but not CMG2−/− mice. Arrows show examples of degenerated hepatocytes with cytosol vacuolization changes. Scale bar, 50 μm. b, Haematoxylin and eosin staining of heart and liver from wild-type (n = 4) and CMG2−/− (n = 3) mice 18 h after 50 μg ET injection (intravenously). In liver, regions with hepatocyte necrotic changes were identified in ET-treated wild-type mice but not CMG2−/− mice. Arrows show necrotic regions, arrowheads indicate examples of intact hepatocytes remaining in the necrotic regions. In heart, only scattered degenerated cardiomyocytes (arrow) were found in ET-treated wild-type but not CMG2−/− mice. Scale bar, 50 μm.
Extended Data Figure 6 Endothelial-cell-specific CMG2-null mice are sensitive to B. anthracis infection.
CMG2(EC)−/− mice and their littermate heterozygous mice were subcutaneously infected with 4 × 108 Sterne spores and monitored for survival. The right panel shows the disease progression of the challenged mice. Log-rank test.
Extended Data Figure 7 LT targeting of liver does not contribute to lethality.
a, Susceptibility of the hepatocyte-specific CMG2-null mice to LT. CMG2(Hep)−/− mice and their littermate CMG2+/+ control mice were challenged intraperitoneally with 100 μg LT and monitored for survival. Whole-body CMG2−/− mice were included as additional controls. b, Selective activation of CMG2 transgene in liver of CMG2Hep mice. Representative fluorescence microscopy of the liver (1), heart (3), skeletal muscles (4), lung (5), small intestines (smooth muscle) (6), aorta (7), spleen (8) and kidney (cortex) (9) from CMG2Hep mice (n = 2), and liver (2) from LSL-CMG2 mice (n = 2). Selective loss of GFP expression in liver from CMG2Hep mice but not LSL-CMG2 mice (1 and 2) is shown. Scale bar, 100 μm. c, Susceptibility of the hepatocyte-specific CMG2-expressing mice to LT. CMG2Hep mice and various control mice as indicated were intraperitoneally challenged with two doses of 100 μg LT and monitored for survival or signs of malaise. Right panel: disease progression of the challenged mice.
Extended Data Figure 8 Oedema in ET-treated mice.
a, ET-induced footpad skin oedema in mice. Mice with various genotypes were injected with 0.25 μg ET (in 20 μl PBS) and the thicknesses of footpads were measured at 0, 8 and 20 h after injection. ET only induced modest oedema in CMG2−/− mice, but caused much higher levels of oedema in CMG2+/−, CMG2(EC)−/−, CMG2(SM/CM)−/− and CMG2(SM/CM/EC)−/− mice. The P values of the indicated groups versus CMG2+/− control group are shown. Each symbol represents one mouse. b–f, ET does not cause oedema in heart, spleen, kidney, lung and brain. Mice were challenged intravenously with 30 μg ET or PBS, and hearts (b), spleens (c), kidneys (d), brains (e) and lungs (f) were collected at 6 h or 18 h for tissue wet/dry ratio measurements. The P values of the indicated groups versus the PBS control group are shown. No significant differences were detected among the groups in b–e. In f, decreases in wet/dry ratio of lungs (dehydration) from ET-treated mice were observed. Each symbol represents one mouse. In a–e, error bars indicate s.e.; two-tailed unpaired t-test.
Extended Data Figure 9 Mice with CMG2 deletion in cardiovascular system and intestines remain sensitive to ET.
a, b, Sensitivity of CMG2(CM)−/−, CMG2(SM/CM/EC)−/−, CMG2(SM/CM/EC/IE)−/− and their littermate control mice to ET. Mice were challenged intravenously with 25 μg ET (a) or intraperitoneally with 50 μg ET (b) and survival monitored after challenge.
Rights and permissions
About this article
Cite this article
Liu, S., Zhang, Y., Moayeri, M. et al. Key tissue targets responsible for anthrax-toxin-induced lethality. Nature 501, 63–68 (2013). https://doi.org/10.1038/nature12510
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12510
This article is cited by
-
Anthrax toxins regulate pain signaling and can deliver molecular cargoes into ANTXR2+ DRG sensory neurons
Nature Neuroscience (2022)
-
Multiple stages of evolutionary change in anthrax toxin receptor expression in humans
Nature Communications (2021)
-
A luminous off-on probe for the determination of 2,6-pyridinedicarboxylic acid as an anthrax biomarker based on water-soluble cadmium sulfide quantum dots
Microchimica Acta (2020)
-
TEM8 functions as a receptor for uPA and mediates uPA-stimulated EGFR phosphorylation
Cell Communication and Signaling (2018)
-
Whole-organism phenotypic screening for anti-infectives promoting host health
Nature Chemical Biology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.