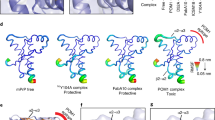
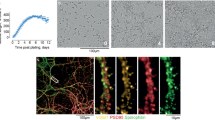
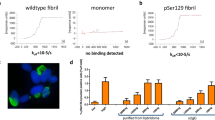
Abstract
Prion infections cause lethal neurodegeneration. This process requires the cellular prion protein (PrPC; ref. 1), which contains a globular domain hinged to a long amino-proximal flexible tail2. Here we describe rapid neurotoxicity in mice and cerebellar organotypic cultured slices exposed to ligands targeting the α1 and α3 helices of the PrPC globular domain. Ligands included seven distinct monoclonal antibodies3, monovalent Fab1 fragments and recombinant single-chain variable fragment miniantibodies. Similar to prion infections4,5,6, the toxicity of globular domain ligands required neuronal PrPC, was exacerbated by PrPC overexpression, was associated with calpain activation and was antagonized by calpain inhibitors. Neurodegeneration was accompanied by a burst of reactive oxygen species, and was suppressed by antioxidants. Furthermore, genetic ablation of the superoxide-producing enzyme NOX2 (also known as CYBB) protected mice from globular domain ligand toxicity. We also found that neurotoxicity was prevented by deletions of the octapeptide repeats within the flexible tail. These deletions did not appreciably compromise globular domain antibody binding, suggesting that the flexible tail is required to transmit toxic signals that originate from the globular domain and trigger oxidative stress and calpain activation. Supporting this view, various octapeptide ligands were not only innocuous to both cerebellar organotypic cultured slices and mice, but also prevented the toxicity of globular domain ligands while not interfering with their binding. We conclude that PrPC consists of two functionally distinct modules, with the globular domain and the flexible tail exerting regulatory and executive functions, respectively. Octapeptide ligands also prolonged the life of mice expressing the toxic PrPC mutant7, PrP(Δ94–134), indicating that the flexible tail mediates toxicity in two distinct PrPC-related conditions. Flexible tail-mediated toxicity may conceivably play a role in further prion pathologies, such as familial Creutzfeldt-Jakob disease in humans bearing supernumerary octapeptides.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
04 September 2013
Minor changes were made to the Acknowledgements.
References
Brandner, S. et al. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature 379, 339–343 (1996)
Riek, R. et al. NMR structure of the mouse prion protein domain PrP(121–231). Nature 382, 180–182 (1996)
Polymenidou, M. et al. The POM monoclonals: a comprehensive set of antibodies to non-overlapping prion protein epitopes. PLoS ONE 3, e3872 (2008)
Falsig, J. et al. Prion pathogenesis is faithfully reproduced in cerebellar organotypic slice cultures. PLoS Pathog. 8, e1002985 (2012)
Fischer, M. et al. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 15, 1255–1264 (1996)
Mallucci, G. et al. Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science 302, 871–874 (2003)
Baumann, F. et al. Lethal recessive myelin toxicity of prion protein lacking its central domain. EMBO J. 26, 538–547 (2007)
Aguzzi, A. & Calella, A. M. Prions: protein aggregation and infectious diseases. Physiol. Rev. 89, 1105–1152 (2009)
Falsig, J. & Aguzzi, A. The prion organotypic slice culture assay—POSCA. Nature Protocols 3, 555–562 (2008)
Riek, R., Hornemann, S., Wider, G., Glockshuber, R. & Wüthrich, K. NMR characterization of the full-length recombinant murine prion protein, mPrP(23–231). FEBS Lett. 413, 282–288 (1997)
Solforosi, L. et al. Cross-linking cellular prion protein triggers neuronal apoptosis in vivo. Science 303, 1514–1516 (2004)
Büeler, H. et al. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 356, 577–582 (1992)
Zahn, R. et al. NMR solution structure of the human prion protein. Proc. Natl Acad. Sci. USA 97, 145–150 (2000)
Wüthrich, K. & Riek, R. Three-dimensional structures of prion proteins. Adv. Protein Chem. 57, 55–82 (2001)
Flechsig, E. et al. Prion protein devoid of the octapeptide repeat region restores susceptibility to scrapie in PrP knockout mice. Neuron 27, 399–408 (2000)
Bremer, J. et al. Axonal prion protein is required for peripheral myelin maintenance. Nature Neurosci. 13, 310–318 (2010)
Wang, K. K. Calpain and caspase: can you tell the difference? Trends Neurosci. 23, 20–26 (2000)
Fatokun, A. A., Stone, T. W. & Smith, R. A. Oxidative stress in neurodegeneration and available means of protection. Front. Biosci. 13, 3288–3311 (2008)
Sorce, S. & Krause, K. H. NOX enzymes in the central nervous system: from signaling to disease. Antioxid. Redox Signal. 11, 2481–2504 (2009)
Silva, A. C., Lee, J. H., Aoki, I. & Koretsky, A. P. Manganese-enhanced magnetic resonance imaging (MEMRI): methodological and practical considerations. NMR Biomed. 17, 532–543 (2004)
Granados-Principal, S., Quiles, J. L., Ramirez-Tortosa, C. L., Sanchez-Rovira, P. & Ramirez-Tortosa, M. C. Hydroxytyrosol: from laboratory investigations to future clinical trials. Nutr. Rev. 68, 191–206 (2010)
Pollock, J. D. et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nature Genet. 9, 202–209 (1995)
Yamaguchi, Y. et al. Biological and biochemical characterization of mice expressing prion protein devoid of the octapeptide repeat region after infection with prions. PLoS ONE 7, e43540 (2012)
Chiesa, R., Piccardo, P., Ghetti, B. & Harris, D. A. Neurological illness in transgenic mice expressing a prion protein with an insertional mutation. Neuron 21, 1339–1351 (1998)
Krasemann, S. et al. Prion disease associated with a novel nine octapeptide repeat insertion in the PRNP gene. Brain Res. Mol. Brain Res. 34, 173–176 (1995)
Mead, S. et al. Inherited prion disease with six octapeptide repeat insertional mutation–molecular analysis of phenotypic heterogeneity. Brain 129, 2297–2317 (2006)
Vital, C., Gray, F., Vital, A., Ferrer, X. & Julien, J. Prion disease with octapeptide repeat insertion. Clin. Exp. Pathol. 47, 153–159 (1999)
Meier, P. et al. Soluble dimeric prion protein binds PrPScin vivo and antagonizes prion disease. Cell 113, 49–60 (2003)
Zahn, R., von Schroetter, C. & Wüthrich, K. Human prion proteins expressed in Escherichia coli and purified by high- affinity column refolding. FEBS Lett. 417, 400–404 (1997)
Lysek, D. A. & Wuthrich, K. Prion protein interaction with the C-terminal SH3 domain of Grb2 studied using NMR and optical spectroscopy. Biochemistry 43, 10393–10399 (2004)
Aller, M. I. et al. Cerebellar granule cell Cre recombinase expression. Genesis 36, 97–103 (2003)
Mallucci, G. R. et al. Post-natal knockout of prion protein alters hippocampal CA1 properties, but does not result in neurodegeneration. EMBO J. 21, 202–210 (2002)
Radovanovic, I. et al. Truncated prion protein and Doppel are myelinotoxic in the absence of oligodendrocytic PrPC. J. Neurosci. 25, 4879–4888 (2005)
Prinz, M. et al. Intrinsic resistance of oligodendrocytes to prion infection. J. Neurosci. 24, 5974–5981 (2004)
Heppner, F. L. et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nature Med. 11, 146–152 (2005)
Levites, Y. et al. Intracranial adeno-associated virus-mediated delivery of anti-pan amyloid β, amyloid β40, and amyloid β42 single-chain variable fragments attenuates plaque pathology in amyloid precursor protein mice. J. Neurosci. 26, 11923–11928 (2006)
Grünecker, B. et al. Fractionated manganese injections: effects on MRI contrast enhancement and physiological measures in C57BL/6 mice. NMR Biomed. 23, 913–921 (2010)
Faas, H. et al. Context-dependent perturbation of neural systems in transgenic mice expressing a cytosolic prion protein. Neuroimage 49, 2607–2617 (2010)
Falsig, J. et al. A versatile prion replication assay in organotypic brain slices. Nature Neurosci. 11, 109–117 (2008)
Das, D., Allen, T. M. & Suresh, M. R. Comparative evaluation of two purification methods of anti-CD19-c-myc-His6-Cys scFv. Protein Expr. Purif. 39, 199–208 (2005)
Baudino, L. et al. IgM and IgA anti-erythrocyte autoantibodies induce anemia in a mouse model through multivalency-dependent hemagglutination but not through complement activation. Blood 109, 5355–5362 (2007)
Hornemann, S., Christen, B., von Schroetter, C., Perez, D. R. & Wuthrich, K. Prion protein library of recombinant constructs for structural biology. FEBS J. 276, 2359–2367 (2009)
Pervushin, K., Riek, R., Wider, G. & Wuthrich, K. Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc. Natl Acad. Sci. USA 94, 12366–12371 (1997)
Keller, R. L. J. The Computer-aided Resonance Assignment Tutorial CARA (Cantina Verlag. 2004) (2004)
Riek, R. NMR of the mouse prion protein, PhD thesis, ETH Zürich (1998)
Baral, P. K. et al. Crystallization and preliminary X-ray diffraction analysis of prion protein bound to the Fab fragment of the POM1 antibody. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 67, 1211–1213 (2011)
Cohen, A. E., Ellis, P. J., Miller, M. D., Deacon, A. M. & Phizackerley, R. P. An automated system to mount cryo-cooled protein crystals on a synchrotron beamline, using compact sample cassettes and a small-scale robot. J. Appl. Crystallogr. 35, 720–726 (2002)
González, A. et al. Web-Ice: integrated data collection and analysis for macromolecular crystallography. J. Appl. Crystallogr. 41, 176–184 (2008)
McPhillips, T. M. et al. Blu-Ice and the Distributed Control System: software for data acquisition and instrument control at macromolecular crystallography beamlines. J. Synchrotron Radiat. 9, 401–406 (2002)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Vagin, A. & Teplyakov, A. MOLREP: an automated program for molecular replacement. J. Appl. Crystallogr. 30, 1022–1025 (1997)
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D 67, 235–242 (2011)
Echols, N. et al. Graphical tools for macromolecular crystallography in PHENIX. J. Appl. Crystallogr. 45, 581–586 (2012)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010)
Acknowledgements
We thank S. Izui, H. Monyer, D. Burton and G. Mallucci for reagents and mice, S. Schauer and the Functional Genomics Center Zurich for advice and help with affinity determinations, A. Steingötter, U. Ungethüm, M. Polymenidou, A. Lau and A. Keller for input, R. Moos, B. Sikorska, C. Tiberi, P. Schwarz, A. Varol, K. Arroyo and M. Delic for technical help. A.A. is the recipient of an Advanced Grant of the European Research Council and is supported by grants from the European Union (PRIORITY, LUPAS and NEURINOX), the Swiss National Foundation, the Foundation Alliance BioSecure, the Clinical Research Priority Program (KFSP) of the University of Zurich, and the Novartis Research Foundation. J.F. is supported by a career development award of the University of Zurich. Research support from PrioNet Canada and Alberta Prion Research Institute (APRI) for the work conducted in the Canadian laboratories is gratefully acknowledged. P.P.L. and A.A. are supported by Polish Swiss Research grant nr PSPB-062/2010. This paper is dedicated to the memory of Dr Marek Fischer, who created the tga20 mouse line.
Author information
Authors and Affiliations
Contributions
T.S., J.F. and A.A. conceived the study. Planning and execution were performed by T.S. with significant contributions from R.R.R., J.F. and additional contribution from T.O’C., S.H., S.Y., B.L. and U.S.H. MEMRI was established and performed by R.R.R.; electron microscopy was performed by P.P.L.; scFv were cloned and produced by B.W., M.S., M.H.R., D.D. and N.K.; X-ray crystallography was performed by P.K.B. and M.N.G.J.; and NMR experiments were performed by S.H. with additional contributions from R.R. T.S., J.F. and A.A. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-4 and Supplementary Figures 1- 15. (PDF 4156 kb)
Three-dimensional representation of the complex between rmPrP120-231 and F(ab)1POM1
Video derived from the structure of its crystal. The rotating image visualizes a discontinuous epitope on PrP with interactions involving both the β1-α1 loop and helix α3. (WMV 5916 kb)
Rights and permissions
About this article
Cite this article
Sonati, T., Reimann, R., Falsig, J. et al. The toxicity of antiprion antibodies is mediated by the flexible tail of the prion protein. Nature 501, 102–106 (2013). https://doi.org/10.1038/nature12402
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12402
This article is cited by
-
Vaccines for prion diseases: a realistic goal?
Cell and Tissue Research (2023)
-
Prion strains viewed through the lens of cryo-EM
Cell and Tissue Research (2023)
-
Mechanisms of prion-induced toxicity
Cell and Tissue Research (2023)
-
A conformational switch controlling the toxicity of the prion protein
Nature Structural & Molecular Biology (2022)
-
Brain aging is faithfully modelled in organotypic brain slices and accelerated by prions
Communications Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.