Abstract
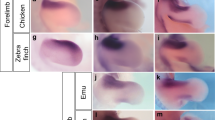
Evolution involves interplay between natural selection and developmental constraints1,2,3. This is seen, for example, when digits are lost from the limbs during evolution1,3,4. Extant archosaurs (crocodiles and birds) show several instances of digit loss3,5,6 under different selective regimes, and show limbs with one, two, three, four or the ancestral number of five digits. The ‘lost’ digits sometimes persist for millions of years as developmental vestiges7,8,9,10. Here we examine digit loss in the Nile crocodile and five birds, using markers of three successive stages of digit development. In two independent lineages under different selection, wing digit I and all its markers disappear. In contrast, hindlimb digit V persists in all species sampled, both as cartilage, and as Sox9- expressing precartilage domains, 250 million years after the adult digit disappeared. There is therefore a mismatch between evolution of the embryonic and adult phenotypes. All limbs, regardless of digit number, showed similar expression of sonic hedgehog (Shh). Even in the one-fingered emu wing, expression of posterior genes Hoxd11 and Hoxd12 was conserved, whereas expression of anterior genes Gli3 and Alx4 was not. We suggest that the persistence of digit V in the embryo may reflect constraints, particularly the conserved posterior gene networks associated with the zone of polarizing activity (ZPA11). The more rapid and complete disappearance of digit I may reflect its ZPA-independent specification, and hence, weaker developmental constraints. Interacting with these constraints are selection pressures for limb functions such as flying and perching. This model may help to explain the diverse patterns of digit loss in tetrapods. Our study may also help to understand how selection on adults leads to changes in development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
06 August 2013
Minor changes were made to the first section of the online Methods.
References
Alberch, P. & Gale, E. A. Size dependence during the development of the amphibian foot: colchicine-induced digital loss and reduction. J. Embryol. Exp. Morphol. 76, 177–197 (1983)
Richardson, M. K. & Chipman, A. D. Developmental constraints in a comparative framework: a test case using variations in phalanx number during amniote evolution. J. Exp. Zool. B Mol. Dev. Evol. 296, 8–22 (2003)
Shapiro, M. D., Shubin, N. H. & Downs, J. P. In Fins Into Limbs: Evolution, Development and Transformation (ed. Hall, B. K. ) 225–244 (Univ. of Chicago Press, 2007)
Alberch, P. & Gale, E. A. A developmental analysis of an evolutionary trend: digital reduction in amphibians. Evolution 39, 8–23 (1985)
Müller, G. B. & Alberch, P. Ontogeny of the limb skeleton in Alligator mississippiensis: developmental invariance and change in the evolution of archosaur limbs. J. Morphol. 203, 151–164 (1990)
Richardson, M. K. in From Clone to Bone: The Synergy of Morphological and Molecular Tools in Palaeobiology 328–362 (eds Asher, R. J. & Müller, J. ) (Cambridge Univ. Press, 2012)
Larsson, H. C. & Wagner, G. P. Pentadactyl ground state of the avian wing. J. Exp. Zool. B Mol. Dev. Evol. 294, 146–151 (2002)
Feduccia, A. & Nowicki, J. The hand of birds revealed by early ostrich embryos. Naturwissenschaften 89, 391–393 (2002)
Welten, M. C., Verbeek, F. J., Meijer, A. H. & Richardson, M. K. Gene expression and digit homology in the chicken embryo wing. Evol. Dev. 7, 18–28 (2005)
Kundrát, M. Primary chondrification foci in the wing basipodium of Struthio camelus with comments on interpretation of autopodial elements in Crocodilia and Aves. J. Exp. Zoolog. B Mol. Dev. Evol. 312, 30–41 (2009)
Harfe, B. D. Keeping up with the zone of polarizing activity: new roles for an old signaling center. Dev. Dyn. 240, 915–919 (2011)
Abourachid, A. & Renous, S. Bipedal locomotion in ratites (Paleognatiform): examples of cursorial birds. Ibis 142, 538–549 (2000)
Schaller, N. U., D'Aout, K., Villa, R., Herkner, B. & Aerts, P. Toe function and dynamic pressure distribution in ostrich locomotion. J. Exp. Biol. 214, 1123–1130 (2011)
Van den berg, C. & Rayner, J. M. V. The moment of inertia of bird wings and the inertial power requirement for flapping flight. J. Exp. Biol. 198, 1655–1664 (1995)
Haddrath, O. & Baker, A. J. Multiple nuclear genes and retroposons support vicariance and dispersal of the palaeognaths, and an Early Cretaceous origin of modern birds. Proc. R. Soc. B 279, 4617–4625 (2012)
Maxwell, E. E. & Larsson, H. C. Osteology and myology of the wing of the Emu (Dromaius novaehollandiae), and its bearing on the evolution of vestigial structures. J. Morphol. 268, 423–441 (2007)
Nagai, H. et al. Embryonic development of the Emu, Dromaius novaehollandiae. Dev. Dyn. 240, 162–175 (2011)
Middleton, K. M. The morphological basis of hallucal orientation in extant birds. J. Morphol. 250, 51–60 (2001)
Sears, K. E. et al. Developmental basis of mammalian digit reduction: a case study in pigs. Evol. Dev. 13, 533–541 (2011)
Lorda-Diez, C. I., Montero, J. A., Diaz-Mendoza, M. J., Garcia-Porrero, J. A. & Hurle, J. M. Defining the earliest transcriptional steps of chondrogenic progenitor specification during the formation of the digits in the embryonic limb. PLoS ONE 6, e24546 (2011)
Hugall, A. F., Foster, R. & Lee, M. S. Y. Calibration choice, rate smoothing, and the pattern of tetrapod diversification according to the long nuclear gene RAG-1. Syst. Biol. 56, 543–563 (2007)
Scherz, P. J., McGlinn, E., Nissim, S. & Tabin, C. J. Extended exposure to Sonic hedgehog is required for patterning the posterior digits of the vertebrate limb. Dev. Biol. 308, 343–354 (2007)
Zhu, J. et al. Uncoupling Sonic hedgehog control of pattern and expansion of the developing limb bud. Dev. Cell 14, 624–632 (2008)
Shapiro, M. D., Hanken, J. & Rosenthal, N. Developmental basis of evolutionary digit loss in the Australian lizard Hemiergis. J. Exp. Zool. B Mol. Dev. Evol. 297, 48–56 (2003)
Gillis, J. A., Dahn, R. D. & Shubin, N. H. Shared developmental mechanisms pattern the vertebrate gill arch and paired fin skeletons. Proc. Natl Acad. Sci. USA 106, 5720–5724 (2009)
Sheth, R. et al. Hox genes regulate digit patterning by controlling the wavelength of a Turing-type mechanism. Science 338, 1476–1480 (2012)
Pagan, S. M., Ros, M. A., Tabin, C. & Fallon, J. F. Surgical removal of limb bud Sonic hedgehog results in posterior skeletal defects. Dev. Biol. 180, 35–40 (1996)
Crumly, C. R. & Sánchez-Villagra, M. R. Patterns of variation in the phalangeal formulae of land tortoises (Testudinidae): developmental constraint, size, and phylogenetic history. J. Exp. Zool. B Mol. Dev. Evol. 302, 134–146 (2004)
Richardson, M. K. Vertebrate evolution: the developmental origins of adult variation. Bioessays 21, 604–613 (1999)
Hamburger, V. & Hamilton, H. L. A series of normal stages in the development of the chick embryo. J. Morphol. 88, 49–92 (1951)
Acknowledgements
We thank M. C. M. Welten for technical advice; W. Bruins for help with collecting the emu embryos; D. van der Marel for taking the radiographs; H. J. Meijer for advice on the phylogeny and for guiding D.A.F. through the the collections at the Smithsonian Institution; the Delaware Museum of Natural History for allowing D.A.F. to measure their specimens; and P. den Hartog, H. Koolmoes and S. de Schaaf-Timmerman for collecting the zebra finch and Barbary dove eggs.
Author information
Authors and Affiliations
Contributions
M.A.G.d.B. conceived the research, wrote the paper, carried out probe design and synthesis, embryo harvesting, gene-expression studies and analysis, and created the figures; D.A.F. carried out gene expression studies, embryo harvesting, and created the adult skeleton figures; K.d.O. carried out gene expression studies and embryo collection; E.M.D. carried out gene-expression studies and collected embryos; M.C.G.-N. carried out alcian blue staining and collected embryos; J.O.H. carried out emu and ostrich embryo collection; D.S. carried out emu embryo collection and incubation; J.-Y.S. carried out crocodile embryo collection and incubation; and M.K.R. conceived the research, wrote the paper, carried out embryo collection and analysis, created figures and provided funding and facilities.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-6, Supplementary Tables 1-3 and Supplementary References. (PDF 2520 kb)
Rights and permissions
About this article
Cite this article
de Bakker, M., Fowler, D., Oude, K. et al. Digit loss in archosaur evolution and the interplay between selection and constraints. Nature 500, 445–448 (2013). https://doi.org/10.1038/nature12336
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12336
This article is cited by
-
‘Dinosaur-bird’ macroevolution, locomotor modules and the origins of flight
Journal of Iberian Geology (2021)
-
Evolutionary Teratology and the Path to Break Through the Mould of the Synthesis Paradigm
Current Molecular Biology Reports (2020)
-
A single-cell transcriptomic atlas of the developing chicken limb
BMC Genomics (2019)
-
Evolution of the avian digital pattern
Scientific Reports (2019)
-
Evidence against tetrapod-wide digit identities and for a limited frame shift in bird wings
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.